Details of the Drug
General Information of Drug (ID: DM1WXA6)
| Drug Name |
Lofexidine
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Britlofex; Lofexidina; Lofexidinum; Britlofex (TN); Lofexidina [INN-Spanish]; Lofexidine (INN); Lofexidine [INN:BAN]; Lofexidinum [INN-Latin]; 1H-Imidazole, 2-(1-(2,6-dichlorophenoxy)ethyl)-4,5-dihydro-(9CI); 2-(1-(2,6-Dichlorophenoxy)ethyl)-4,5-dihydro-1H-imidazole; 2-(a-(2,6-dichlorophenoxy)ethyl)2-imidazoline; 2-(alpha-(2,6-Dichlorophenoxy)ethyl)2-imidazoline; 2-(alpha-(2,6-dichlorophenoxy)ethyl) delta-2-imidazoline; 2-[1-(2,6-dichlorophenoxy)ethyl]-4,5-dihydro-1H-imidazole; 2-{1-[(2,6-dichlorophenyl)oxy]ethyl}-4,5-dihydro-1H-imidazole
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Antihypertensive Agents
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
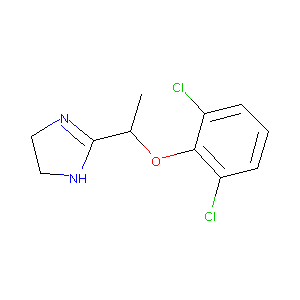 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 259.13 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.6 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Lofexidine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | A Phase 3 placebo-controlled, double-blind, multi-site trial of the alpha-2-adrenergic agonist, lofexidine, for opioid withdrawal. Drug Alcohol Depend. 2008 Sep 1;97(1-2):158-68. | ||||
|---|---|---|---|---|---|
| 2 | 2018 FDA drug approvals.Nat Rev Drug Discov. 2019 Feb;18(2):85-89. | ||||
| 3 | EMA Public Assessment Report: Hepcludex (bulevirtide) powder for injectable solution | ||||
| 4 | BDDCS predictions, self-correcting aspects of BDDCS assignments, BDDCS assignment corrections, and classification for more than 175 additional drugs | ||||
| 5 | FDA reports | ||||
| 6 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 7 | Clinical pharmacokinetics of lofexidine, the alpha 2-adrenergic receptor agonist, in opiate addicts plasma using a highly sensitive liquid chromatography tandem mass spectrometric analysis. Am J DrugAlcohol Abuse. 2008;34(5):611-6. | ||||
| 8 | Advisory committee lofexidine hydrochloride (lucemyratm) briefing document. | ||||
| 9 | The Drugs.com International Drug Name Database: Lofexidine | ||||
| 10 | Product Information. Tibsovo (ivosidenib). Agios Pharmaceuticals, Cambridge, MA. | ||||
| 11 | Canadian Pharmacists Association. | ||||
| 12 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 13 | Product Information. Xospata (gilteritinib). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 14 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 15 | Product Information. Arcapta Neohaler (indacaterol). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 16 | Product Information. Daurismo (glasdegib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 17 | Bengtsson B, Fagerstrom PO "Extrapulmonary effects of terbutaline during prolonged administration." Clin Pharmacol Ther 31 (1982): 726-32. [PMID: 7042176] | ||||
| 18 | Aronowitz JS, Chakos MH, Safferman AZ, Lieberman JA "Syncope associated with the combination of clozapine and enalapril." J Clin Psychopharmacol 14 (1994): 429-30. [PMID: 7884028] | ||||
| 19 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 20 | Product Information. Rukobia (fostemsavir). ViiV Healthcare, Research Triangle Park, NC. | ||||
| 21 | Product Information. Xalkori (crizotinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 22 | Product Information. Tagrisso (osimertinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 23 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 24 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 25 | Product Information. Farydak (panobinostat). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 26 | Product Information. Zeposia (ozanimod). Celgene Corporation, Summit, NJ. | ||||
| 27 | Ban TA "Drug interactions with psychoactive drugs." Dis Nerv Syst 36 (1975): 164-6. [PMID: 1116424] | ||||
| 28 | Product Information. Nuplazid (pimavanserin). Accelis Pharma, East Windsor, NJ. | ||||
| 29 | Product Information. Macrilen (macimorelin). Aeterna Zentaris, Charleston, SC. | ||||
| 30 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
