| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
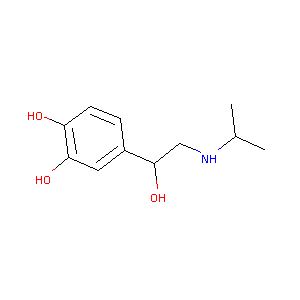
Isoproterenol FDA Label
|
| 3 |
FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 083346.
|
| 4 |
A phase 1 study of AS1409, a novel antibody-cytokine fusion protein, in patients with malignant melanoma or renal cell carcinoma. Clin Cancer Res. 2011 Apr 1;17(7):1998-2005.
|
| 5 |
Management of symptomatic cholelithiasis: a systematic review. Syst Rev. 2022 Dec 12;11(1):267.
|
| 6 |
Primary biliary cholangitis: pathogenesis and therapeutic opportunities. Nat Rev Gastroenterol Hepatol. 2020 Feb;17(2):93-110.
|
| 7 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7104).
|
| 8 |
Design and synthesis of bile acid derivatives and their activity against colon cancer. RSC Med Chem. 2022 Aug 19;13(11):1391-1409.
|
| 9 |
Isoproterenol effects evaluated in heart slices of human and rat in comparison to rat heart in vivo. Toxicol Appl Pharmacol. 2014 Jan 15;274(2):302-12.
|
| 10 |
Current therapeutic uses and potential of beta-adrenoceptor agonists and antagonists. Eur J Clin Pharmacol. 1998 Feb;53(6):389-404.
|
| 11 |
A phase 1 study of AS1409, a novel antibody-cytokine fusion protein, in patients with malignant melanoma or renal cell carcinoma. Clin Cancer Res. 2011 Apr 1;17(7):1998-2005.
|
| 12 |
Identification, characterization, and ontogenic study of a catechol O-methyltransferase from zebrafish. Aquat Toxicol. 2011 Mar;102(1-2):18-23.
|
| 13 |
Role of adrenoceptor-linked signaling pathways in the regulation of CYP1A1 gene expression. Biochem Pharmacol. 2005 Jan 15;69(2):277-87.
|
| 14 |
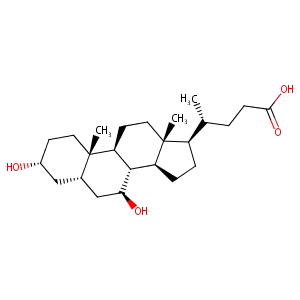
An in vitro coculture system of human peripheral blood mononuclear cells with hepatocellular carcinoma-derived cells for predicting drug-induced liver injury. Arch Toxicol. 2021 Jan;95(1):149-168. doi: 10.1007/s00204-020-02882-4. Epub 2020 Aug 20.
|
| 15 |
Ligand binding and aggregation of pathogenic SOD1. Nat Commun. 2013;4:1758. doi: 10.1038/ncomms2750.
|
| 16 |
Hypokalemia from beta2-receptor stimulation by circulating epinephrine. N Engl J Med. 1983 Dec 8;309(23):1414-9. doi: 10.1056/NEJM198312083092303.
|
| 17 |
Vascular renin-angiotensin system and neurotransmission in hypertensive persons. Hypertension. 1991 Sep;18(3):266-77. doi: 10.1161/01.hyp.18.3.266.
|
| 18 |
The regulation of human vascular smooth muscle extracellular matrix protein production by alpha- and beta-adrenoceptor stimulation. J Hypertens. 2002 Feb;20(2):287-94. doi: 10.1097/00004872-200202000-00019.
|
| 19 |
Low doses of BPF-induced hypertrophy in cardiomyocytes derived from human embryonic stem cells via disrupting the mitochondrial fission upon the interaction between ER and calcineurin A-DRP1 signaling pathway. Cell Biol Toxicol. 2022 Jun;38(3):409-426. doi: 10.1007/s10565-021-09615-y. Epub 2021 May 22.
|
| 20 |
Post-receptorial mechanisms underlie functional disregulation of beta2-adrenergic receptors in lymphocytes from Multiple Sclerosis patients. J Neuroimmunol. 2004 Oct;155(1-2):143-9. doi: 10.1016/j.jneuroim.2004.05.013.
|
| 21 |
Prostaglandin E2 acts at two distinct pathways of T lymphocyte activation: inhibition of interleukin 2 production and down-regulation of transferrin receptor expression. J Immunol. 1985 Aug;135(2):1172-9.
|
| 22 |
The effects of desmethylimipramine on cyclic AMP-stimulated gene transcription in a model cell system. Biochem Pharmacol. 2005 Sep 1;70(5):762-9. doi: 10.1016/j.bcp.2005.06.012.
|
| 23 |
Isoproterenol inhibits angiotensin II-stimulated proliferation and reactive oxygen species production in vascular smooth muscle cells through heme oxygenase-1. Biol Pharm Bull. 2009 Jun;32(6):1047-52. doi: 10.1248/bpb.32.1047.
|
| 24 |
Quercetin-3-O-glucuronide inhibits noradrenaline-promoted invasion of MDA-MB-231 human breast cancer cells by blocking ?-adrenergic signaling. Arch Biochem Biophys. 2014 Sep 1;557:18-27. doi: 10.1016/j.abb.2014.05.030. Epub 2014 Jun 11.
|
| 25 |
Impaired PARP activity in response to the -adrenergic receptor agonist isoproterenol. Toxicol In Vitro. 2018 Aug;50:29-39. doi: 10.1016/j.tiv.2018.02.001. Epub 2018 Feb 10.
|
| 26 |
Activation of airway cl- secretion in human subjects by adenosine. Am J Respir Cell Mol Biol. 2004 Aug;31(2):140-6. doi: 10.1165/rcmb.2004-0012OC. Epub 2004 Mar 23.
|
| 27 |
Discovery of a novel series of biphenyl benzoic acid derivatives as highly potent and selective human beta3 adrenergic receptor agonists with good oral bioavailability. Part II. J Med Chem. 2008 Jul 10;51(13):4002-20. doi: 10.1021/jm8000345. Epub 2008 Jun 14.
|
| 28 |
Adrenoceptor blockade alters plasma gelatinase activity in patients with heart failure and MMP-9 promoter activity in a human cell line (ECV304). Pharmacol Res. 2006 Jul;54(1):57-64. doi: 10.1016/j.phrs.2006.02.006. Epub 2006 Feb 28.
|
| 29 |
Reciprocal in vivo regulation of myocardial G protein-coupled receptor kinase expression by beta-adrenergic receptor stimulation and blockade. Circulation. 1998 Oct 27;98(17):1783-9. doi: 10.1161/01.cir.98.17.1783.
|
| 30 |
A unique mechanism of beta-blocker action: carvedilol stimulates beta-arrestin signaling. Proc Natl Acad Sci U S A. 2007 Oct 16;104(42):16657-62. doi: 10.1073/pnas.0707936104. Epub 2007 Oct 9.
|
| 31 |
Regulation of beta-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell. 2007 May 4;129(3):511-22. doi: 10.1016/j.cell.2007.02.046.
|
| 32 |
Catecholamines suppress leptin release from in vitro differentiated subcutaneous human adipocytes in primary culture via beta1- and beta2-adrenergic receptors. Eur J Endocrinol. 2000 Sep;143(3):439-45. doi: 10.1530/eje.0.1430439.
|
| 33 |
IL-13 and IL-4 promote TARC release in human airway smooth muscle cells: role of IL-4 receptor genotype. Am J Physiol Lung Cell Mol Physiol. 2003 Oct;285(4):L907-14. doi: 10.1152/ajplung.00120.2003. Epub 2003 Jul 18.
|
| 34 |
Carvedilol and its new analogs suppress arrhythmogenic store overload-induced Ca2+ release. Nat Med. 2011 Jul 10;17(8):1003-9. doi: 10.1038/nm.2406.
|
| 35 |
Autocrine interaction between IL-5 and IL-1beta mediates altered responsiveness of atopic asthmatic sensitized airway smooth muscle. J Clin Invest. 1999 Sep;104(5):657-67. doi: 10.1172/JCI7137.
|
| 36 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 37 |
BMP10 preserves cardiac function through its dual activation of SMAD-mediated and STAT3-mediated pathways. J Biol Chem. 2019 Dec 27;294(52):19877-19888. doi: 10.1074/jbc.RA119.010943. Epub 2019 Nov 11.
|
| 38 |
Identification of specific ligands for orphan olfactory receptors. G protein-dependent agonism and antagonism of odorants. J Biol Chem. 2005 Mar 25;280(12):11807-15. doi: 10.1074/jbc.M411508200. Epub 2004 Dec 14.
|
| 39 |
Molecular mechanisms controlling the rate and specificity of catechol O-methylation by human soluble catechol O-methyltransferase. Mol Pharmacol. 2001 Feb;59(2):393-402. doi: 10.1124/mol.59.2.393.
|
| 40 |
Gene expression profiling of early primary biliary cirrhosis: possible insights into the mechanism of action of ursodeoxycholic acid. Liver Int. 2008 Aug;28(7):997-1010. doi: 10.1111/j.1478-3231.2008.01744.x. Epub 2008 Apr 15.
|
| 41 |
UGT-dependent regioselective glucuronidation of ursodeoxycholic acid and obeticholic acid and selective transport of the consequent acyl glucuronides by OATP1B1 and 1B3. Chem Biol Interact. 2019 Sep 1;310:108745. doi: 10.1016/j.cbi.2019.108745. Epub 2019 Jul 9.
|
| 42 |
Role of vitamin C transporters and biliverdin reductase in the dual pro-oxidant and anti-oxidant effect of biliary compounds on the placental-fetal... Toxicol Appl Pharmacol. 2008 Oct 15;232(2):327-36.
|
| 43 |
Transport of fluorescent chenodeoxycholic acid via the human organic anion transporters OATP1B1 and OATP1B3. J Lipid Res. 2006 Jun;47(6):1196-202.
|
| 44 |
Identification, cloning, heterologous expression, and characterization of a NADPH-dependent 7beta-hydroxysteroid dehydrogenase from Collinsella aerofaciens. Appl Microbiol Biotechnol. 2011 Apr;90(1):127-35.
|
| 45 |
Selective and potent inhibitors of human 20alpha-hydroxysteroid dehydrogenase (AKR1C1) that metabolizes neurosteroids derived from progesterone. Chem Biol Interact. 2003 Feb 1;143-144:503-13.
|
| 46 |
Effects of ursodeoxycholic acid on P-glycoprotein and cytochrome P450 3A4-dependent pharmacokinetics in humans. Clin Pharmacol Ther. 2006 May;79(5):449-60.
|
| 47 |
Role of vitamin C transporters and biliverdin reductase in the dual pro-oxidant and anti-oxidant effect of biliary compounds on the placental-fetal unit in cholestasis during pregnancy. Toxicol Appl Pharmacol. 2008 Oct 15;232(2):327-36.
|
| 48 |
Ursodeoxycholic acid but not tauroursodeoxycholic acid inhibits proliferation and differentiation of human subcutaneous adipocytes. PLoS One. 2013 Dec 3;8(12):e82086. doi: 10.1371/journal.pone.0082086. eCollection 2013.
|
| 49 |
Lipid raft-dependent death receptor 5 (DR5) expression and activation are critical for ursodeoxycholic acid-induced apoptosis in gastric cancer cells. Carcinogenesis. 2011 May;32(5):723-31. doi: 10.1093/carcin/bgr038. Epub 2011 Feb 28.
|
| 50 |
Potency of individual bile acids to regulate bile acid synthesis and transport genes in primary human hepatocyte cultures. Toxicol Sci. 2014 Oct;141(2):538-46. doi: 10.1093/toxsci/kfu151. Epub 2014 Jul 23.
|
| 51 |
The synthetic bile acid-phospholipid conjugate ursodeoxycholyl lysophosphatidylethanolamide suppresses TNF-induced liver injury. J Hepatol. 2011 Apr;54(4):674-84. doi: 10.1016/j.jhep.2010.07.028. Epub 2010 Sep 27.
|
| 52 |
The farnesoid X receptor controls gene expression in a ligand- and promoter-selective fashion. J Biol Chem. 2004 Mar 5;279(10):8856-61. doi: 10.1074/jbc.M306422200. Epub 2003 Dec 18.
|
| 53 |
Ligand-independent activation of the glucocorticoid receptor by ursodeoxycholic acid. Repression of IFN-gamma-induced MHC class II gene expression via a glucocorticoid receptor-dependent pathway. J Immunol. 1996 Feb 15;156(4):1601-8.
|
| 54 |
Hepatic apolipoprotein A-I gene expression in patients with cholesterol gallstones treated with ursodeoxycholic acid. Ann Hepatol. 2002 Apr-Jun;1(2):85-9.
|
| 55 |
Combination of ursodeoxycholic acid and glucocorticoids upregulates the AE2 alternate promoter in human liver cells. J Clin Invest. 2008 Feb;118(2):695-709. doi: 10.1172/JCI33156.
|
| 56 |
Ursodeoxycholic acid modulates the ubiquitin-proteasome degradation pathway of p53. Biochem Biophys Res Commun. 2010 Oct 1;400(4):649-54. doi: 10.1016/j.bbrc.2010.08.121. Epub 2010 Aug 31.
|
| 57 |
Ursodeoxycholic acid inhibits endothelin-1 production in human vascular endothelial cells. Eur J Pharmacol. 2004 Nov 28;505(1-3):67-74. doi: 10.1016/j.ejphar.2004.10.042.
|
| 58 |
Expression of protein kinase C isoenzymes in colorectal cancer tissue and their differential activation by different bile acids. Int J Cancer. 1995 Mar 29;61(1):35-9. doi: 10.1002/ijc.2910610107.
|
| 59 |
Mechanism of apoptotic effects induced selectively by ursodeoxycholic acid on human hepatoma cell lines. World J Gastroenterol. 2007 Mar 21;13(11):1652-8. doi: 10.3748/wjg.v13.i11.1652.
|
| 60 |
Correlation of chemopreventive efficacy data from the human epidermal cell assay with in vivo data. Anticancer Res. 2000 Jan-Feb;20(1A):27-32.
|
| 61 |
Ursodeoxycholic acid reduces increased circulating endothelin 2 in primary biliary cirrhosis. Aliment Pharmacol Ther. 2005 Feb 1;21(3):227-34. doi: 10.1111/j.1365-2036.2005.02307.x.
|
| 62 |
Ursodeoxycholic acid induces glutathione synthesis through activation of PI3K/Akt pathway in HepG2 cells. Biochem Pharmacol. 2009 Mar 1;77(5):858-66. doi: 10.1016/j.bcp.2008.11.012. Epub 2008 Nov 25.
|
| 63 |
Regulation of ileal bile acid-binding protein expression in Caco-2 cells by ursodeoxycholic acid: role of the farnesoid X receptor. Biochem Pharmacol. 2005 Jun 15;69(12):1755-63. doi: 10.1016/j.bcp.2005.03.019.
|
| 64 |
Ursodeoxycholic acid-induced inhibition of DLC1 protein degradation leads to suppression of hepatocellular carcinoma cell growth. Oncol Rep. 2011 Jun;25(6):1739-46. doi: 10.3892/or.2011.1239. Epub 2011 Mar 29.
|
| 65 |
Chemiluminescence quantitative immunohistochemical determination of MRP2 in liver biopsies. J Histochem Cytochem. 2005 Dec;53(12):1451-7. doi: 10.1369/jhc.5A6621.2005. Epub 2005 Jun 13.
|
| 66 |
Kinetic analysis of bile acid sulfation by stably expressed human sulfotransferase 2A1 (SULT2A1). Xenobiotica. 2010 Mar;40(3):184-94.
|
|
|
|
|
|
|