Details of the Drug
General Information of Drug (ID: DMK7MEY)
| Drug Name |
Isoproterenol
|
||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
isoproterenol; Isoprenaline; Isoprenalin; Norisodrine; Novodrin; Isopropydrin; Isopropylarterenol; Respifral; Assiprenol; Asiprenol; Bellasthman; Asmalar; Aludrine; Aludrin; N-Isopropylnoradrenaline; Bronkephrine; Neodrenal; Lomupren; Isonorene; Isopropyladrenaline; 7683-59-2; N-Isopropylnorepinephrine; Isopropylnorepinephrine; neo-Epinine; Isadrine; Saventrine; Isorenin; Isonorin; Proternol; Isopropylnoradrenaline; Isopropyl noradrenaline; Racemic isoprenaline; dl-Isadrine; Racemic isoproterenol; (+-)-Isoproterenol; Vapo-N-iso; Aerolone; Aleudrine; Dihydroxyphenylethanolisopropylamine; Euspiran; ISOPROP; Isadrin; Isoproterenolum; Isuprel; Isupren; Izadrin; Epinephrine isopropyl homolog; Isoprenaline hydrochloride; Isoproterenol Chloride; Isoproterenol [JAN]; Isuprel Mistometer; WIN 5162; D-Isoprenaline; D-Isopropylarterenol; D-Isoproterenol; DL-Isopropylnorepinephrine; Dl-Ipr; Dl-Isadrine; Dl-Isopropylnoradrenaline; Isoprenalina [INN-Spanish]; Isoprenaline (INN); Isoprenalinum [INN-Latin]; Isuprel (TN); L-Isopropylnoradrenaline; L-Isoproterenol; Medihaler-ISO; Neo-Epinine; Vapo-Iso; Alpha-(Isopropylaminomethyl)protocatechuyl alcohol; Alpha-(Isopropylaminomoethyl)protocatechuyl alcohol; D-N-Isopropylnorepinephrine; Dl-N-Isopropylnoradrenaline; Isopropylaminomethyl(3,4-dihydroxyphenyl)carbinol; Isopropylaminomethyl-3,4-dihydroxyphenyl carbinol; DL(+-)-Isoproterenol; N-Isopropyl-beta-dihydroxyphenyl-beta-hydroxyethylamine; (+)-Isoprenaline; (+)-Isoproterenol; (+-)-Isoprenaline; (-)-Isoproterenol hydrochloride; (S)-(+)-Isoproterenol; (S)-Isoprenaline; (S)-Isoproterenol; 1,2-Benzenediol, 4-(1-hydroxy-2-((1-methylethyl)amino)ethyl)-(9CI); 1,2-Benzenediol, 4-(1-hydroxy-2-((1-methylethyl)amino)ethyl)-, (S)-(9CI); 1-(3,4-Dihydroxyphenyl)-2-(isopropylamino)ethanol; 1-(3,4-Dihydroxyphenyl)-2-isopropylaminoethanol; 3,4-Dihydroxy-alpha-(isopropylaminomethyl)-benzyl alcohol; 3,4-Dihydroxy-alpha-[(isopropylamino)methyl]benzyl alcohol; 3,4-Dihydroxy-alpha-((isopropylamino)methyl)benzyl alcohol; 4-(1-Hydroxy-2(isopropylamino)ethyl)-benzene 1,2-diol; 4-(1-Hydroxy-2-((1-methylethyl)amino)ethyl)-1,2-benzenediol; 4-{1-hydroxy-2-[(1-methylethyl)amino]ethyl}benzene-1,2-diol; AS1409
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Cardiotonic Agents
|
||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
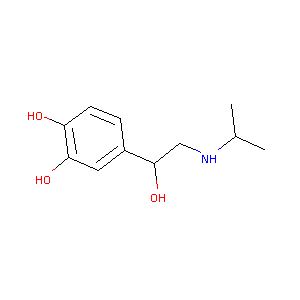 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 211.26 | |||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.6 | ||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Atrioventricular block | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Isoproterenol (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Isoproterenol FDA Label | ||||
|---|---|---|---|---|---|
| 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 083346. | ||||
| 3 | A phase 1 study of AS1409, a novel antibody-cytokine fusion protein, in patients with malignant melanoma or renal cell carcinoma. Clin Cancer Res. 2011 Apr 1;17(7):1998-2005. | ||||
| 4 | Reyes G, Schwartz PH, Newth CJ, Eldadah MK: The pharmacokinetics of isoproterenol in critically ill pediatric patients. J Clin Pharmacol. 1993 Jan;33(1):29-34. doi: 10.1002/j.1552-4604.1993.tb03899.x. | ||||
| 5 | Klimas R, Mikus G: Morphine-6-glucuronide is responsible for the analgesic effect after morphine administration: a quantitative review of morphine, morphine-6-glucuronide, and morphine-3-glucuronide. Br J Anaesth. 2014 Dec;113(6):935-44. doi: 10.1093/bja/aeu186. Epub 2014 Jul 1. | ||||
| 6 | Isoproterenol . | ||||
| 7 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 8 | Current therapeutic uses and potential of beta-adrenoceptor agonists and antagonists. Eur J Clin Pharmacol. 1998 Feb;53(6):389-404. | ||||
| 9 | A phase 1 study of AS1409, a novel antibody-cytokine fusion protein, in patients with malignant melanoma or renal cell carcinoma. Clin Cancer Res. 2011 Apr 1;17(7):1998-2005. | ||||
| 10 | Identification, characterization, and ontogenic study of a catechol O-methyltransferase from zebrafish. Aquat Toxicol. 2011 Mar;102(1-2):18-23. | ||||
| 11 | Isoproterenol effects evaluated in heart slices of human and rat in comparison to rat heart in vivo. Toxicol Appl Pharmacol. 2014 Jan 15;274(2):302-12. | ||||
| 12 | Quercetin-3-O-glucuronide inhibits noradrenaline-promoted invasion of MDA-MB-231 human breast cancer cells by blocking ?-adrenergic signaling. Arch Biochem Biophys. 2014 Sep 1;557:18-27. doi: 10.1016/j.abb.2014.05.030. Epub 2014 Jun 11. | ||||
| 13 | The effects of desmethylimipramine on cyclic AMP-stimulated gene transcription in a model cell system. Biochem Pharmacol. 2005 Sep 1;70(5):762-9. doi: 10.1016/j.bcp.2005.06.012. | ||||
| 14 | Vascular renin-angiotensin system and neurotransmission in hypertensive persons. Hypertension. 1991 Sep;18(3):266-77. doi: 10.1161/01.hyp.18.3.266. | ||||
| 15 | An in vitro coculture system of human peripheral blood mononuclear cells with hepatocellular carcinoma-derived cells for predicting drug-induced liver injury. Arch Toxicol. 2021 Jan;95(1):149-168. doi: 10.1007/s00204-020-02882-4. Epub 2020 Aug 20. | ||||
| 16 | Boakes AJ, Laurence DR, Teoh PC, Barar FS, Benedikter LT, Prichard BN "Interactions between sympathomimetic amines and antidepressant agents in man." Br Med J 1 (1973): 311-5. [PMID: 4685619] | ||||
| 17 | Bengtsson B, Fagerstrom PO "Extrapulmonary effects of terbutaline during prolonged administration." Clin Pharmacol Ther 31 (1982): 726-32. [PMID: 7042176] | ||||
| 18 | Product Information. Arcapta Neohaler (indacaterol). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 19 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 20 | Product Information. Northera (droxidopa). Chelsea Therapeutics Inc, Charlotte, NC. | ||||
| 21 | Product Information. Cymbalta (duloxetine). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 22 | Product Information. Zeposia (ozanimod). Celgene Corporation, Summit, NJ. | ||||
| 23 | Mendelson J, Jones RT, Upton R, Jacob P 3rd "Methamphetamine and ethanol interactions in humans." Clin Pharmacol Ther 57 (1995): 559-68. [PMID: 7768079] | ||||
| 24 | Illi A, Sundberg S, Ojala-Karlsson P, Korhonen P, Scheinin M, Gordin A "The effect of entacapone on the disposition and hemodynamic effects of intravenous isoproterenol and epinephrine." Clin Pharmacol Ther 58 (1995): 221-7. [PMID: 7648772] | ||||
| 25 | Product Information. Fycompa (perampanel). Eisai Inc, Teaneck, NJ. | ||||
| 26 | Canadian Pharmacists Association. | ||||
