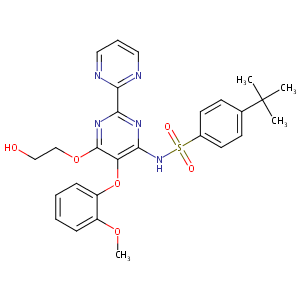
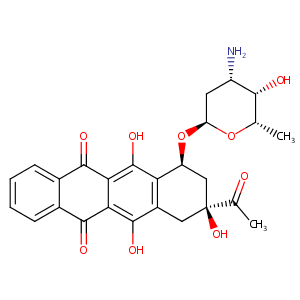
| DOT Name |
DOT ID |
UniProt ID |
Mode of Action |
REF |
|
Cytochrome P450 3A4 (CYP3A4)
|
OTQGYY83
|
CP3A4_HUMAN
|
Increases Expression
|
[11] |
|
Solute carrier organic anion transporter family member 1B1 (SLCO1B1)
|
OTNEN8QK
|
SO1B1_HUMAN
|
Decreases Expression
|
[6] |
|
Bile salt export pump (ABCB11)
|
OTRU7THO
|
ABCBB_HUMAN
|
Decreases Expression
|
[6] |
|
Endothelin-1 (EDN1)
|
OTZCACEG
|
EDN1_HUMAN
|
Decreases Activity
|
[12] |
|
Nitric oxide synthase 3 (NOS3)
|
OTLDT7NR
|
NOS3_HUMAN
|
Increases ADR
|
[13] |
|
Cytochrome P450 2B6 (CYP2B6)
|
OTOYO4S7
|
CP2B6_HUMAN
|
Increases Expression
|
[11] |
|
Serine/threonine-protein kinase DCLK1 (DCLK1)
|
OTVSPS98
|
DCLK1_HUMAN
|
Affects Expression
|
[6] |
|
Kynurenine 3-monooxygenase (KMO)
|
OT3MRMM9
|
KMO_HUMAN
|
Affects Expression
|
[6] |
|
Carbonic anhydrase 12 (CA12)
|
OT6WNFU8
|
CAH12_HUMAN
|
Affects Expression
|
[6] |
|
All-trans-retinol dehydrogenase ADH1B (ADH1B)
|
OTV3TB81
|
ADH1B_HUMAN
|
Decreases Expression
|
[6] |
|
Alcohol dehydrogenase 1C (ADH1C)
|
OTO1DV7F
|
ADH1G_HUMAN
|
Decreases Expression
|
[6] |
|
Superoxide dismutase (SOD1)
|
OT39TA1L
|
SODC_HUMAN
|
Decreases Expression
|
[6] |
|
Complement component C9 (C9)
|
OT7I5FDX
|
CO9_HUMAN
|
Affects Expression
|
[6] |
|
Catalase (CAT)
|
OTHEBX9R
|
CATA_HUMAN
|
Decreases Expression
|
[6] |
|
Superoxide dismutase , mitochondrial (SOD2)
|
OTIWXGZ9
|
SODM_HUMAN
|
Increases Expression
|
[6] |
|
Insulin-like growth factor I (IGF1)
|
OTIIZR61
|
IGF1_HUMAN
|
Affects Expression
|
[6] |
|
Fructose-bisphosphate aldolase B (ALDOB)
|
OT7FR69A
|
ALDOB_HUMAN
|
Decreases Expression
|
[6] |
|
Interleukin-6 (IL6)
|
OTUOSCCU
|
IL6_HUMAN
|
Increases Expression
|
[6] |
|
Fatty acid-binding protein, liver (FABP1)
|
OTR34ETM
|
FABPL_HUMAN
|
Decreases Expression
|
[6] |
|
Glutathione peroxidase 1 (GPX1)
|
OTE2O72Q
|
GPX1_HUMAN
|
Decreases Expression
|
[6] |
|
Alcohol dehydrogenase 1A (ADH1A)
|
OTTS983E
|
ADH1A_HUMAN
|
Decreases Expression
|
[6] |
|
Amino acid transporter heavy chain SLC3A2 (SLC3A2)
|
OTBR33M9
|
4F2_HUMAN
|
Increases Expression
|
[6] |
|
All-trans-retinol dehydrogenase ADH4 (ADH4)
|
OTKDKKNG
|
ADH4_HUMAN
|
Decreases Expression
|
[6] |
|
Heme oxygenase 1 (HMOX1)
|
OTC1W6UX
|
HMOX1_HUMAN
|
Increases Expression
|
[6] |
|
Glutathione S-transferase theta-2 (GSTT2)
|
OTANW3TJ
|
GST2_HUMAN
|
Decreases Expression
|
[6] |
|
Interleukin-8 (CXCL8)
|
OTS7T5VH
|
IL8_HUMAN
|
Increases Expression
|
[6] |
|
Microsomal glutathione S-transferase 1 (MGST1)
|
OTGN1KVZ
|
MGST1_HUMAN
|
Decreases Expression
|
[6] |
|
3 beta-hydroxysteroid dehydrogenase/Delta 5-->4-isomerase type 1 (HSD3B1)
|
OTNAZVKB
|
3BHS1_HUMAN
|
Affects Expression
|
[6] |
|
NAD(P)H dehydrogenase 1 (NQO1)
|
OTZGGIVK
|
NQO1_HUMAN
|
Increases Expression
|
[6] |
|
NADPH--cytochrome P450 reductase (POR)
|
OTVIDOCH
|
NCPR_HUMAN
|
Affects Expression
|
[6] |
|
Tyrosine aminotransferase (TAT)
|
OT2CJ91O
|
ATTY_HUMAN
|
Affects Expression
|
[6] |
|
Phosphatidylcholine translocator ABCB4 (ABCB4)
|
OTE6PY83
|
MDR3_HUMAN
|
Decreases Expression
|
[6] |
|
Cytochrome P450 7A1 (CYP7A1)
|
OT8Z5KLD
|
CP7A1_HUMAN
|
Decreases Expression
|
[6] |
|
Macrophage mannose receptor 1 (MRC1)
|
OTTVCOPT
|
MRC1_HUMAN
|
Affects Expression
|
[6] |
|
Cytochrome P450 3A7 (CYP3A7)
|
OTTCDHHM
|
CP3A7_HUMAN
|
Affects Expression
|
[6] |
|
Serum paraoxonase/arylesterase 1 (PON1)
|
OTD0Z2XO
|
PON1_HUMAN
|
Decreases Expression
|
[6] |
|
G0/G1 switch protein 2 (G0S2)
|
OT8FL49L
|
G0S2_HUMAN
|
Affects Expression
|
[6] |
|
Sodium- and chloride-dependent GABA transporter 1 (SLC6A1)
|
OTLCJJF3
|
SC6A1_HUMAN
|
Affects Expression
|
[6] |
|
Pyruvate kinase PKLR (PKLR)
|
OTTTM1QI
|
KPYR_HUMAN
|
Affects Expression
|
[6] |
|
Carbamoyl-phosphate synthase , mitochondrial (CPS1)
|
OTXV8NSR
|
CPSM_HUMAN
|
Decreases Expression
|
[6] |
|
Glucose-6-phosphatase catalytic subunit 1 (G6PC1)
|
OTJ6FM9F
|
G6PC1_HUMAN
|
Affects Expression
|
[6] |
|
Peroxisome proliferator-activated receptor gamma (PPARG)
|
OTHMARHO
|
PPARG_HUMAN
|
Decreases Expression
|
[6] |
|
Hepatocyte nuclear factor 4-alpha (HNF4A)
|
OTY1TOAB
|
HNF4A_HUMAN
|
Decreases Expression
|
[6] |
|
Glutamate--cysteine ligase catalytic subunit (GCLC)
|
OTESDI4D
|
GSH1_HUMAN
|
Increases Expression
|
[6] |
|
Sulfotransferase 1E1 (SULT1E1)
|
OTGPJ517
|
ST1E1_HUMAN
|
Affects Expression
|
[6] |
|
Achaete-scute homolog 1 (ASCL1)
|
OTI4X44G
|
ASCL1_HUMAN
|
Affects Expression
|
[6] |
|
Oxysterols receptor LXR-beta (NR1H2)
|
OT4APA60
|
NR1H2_HUMAN
|
Decreases Expression
|
[6] |
|
Microsomal triglyceride transfer protein large subunit (MTTP)
|
OTNUVSDT
|
MTP_HUMAN
|
Decreases Expression
|
[6] |
|
Inhibin beta E chain (INHBE)
|
OTOI2NYG
|
INHBE_HUMAN
|
Affects Expression
|
[6] |
|
Metallothionein-1X (MT1X)
|
OT9AKFVS
|
MT1X_HUMAN
|
Increases Expression
|
[6] |
|
Complement factor H-related protein 3 (CFHR3)
|
OTYL8SDO
|
FHR3_HUMAN
|
Affects Expression
|
[6] |
|
Oxysterols receptor LXR-alpha (NR1H3)
|
OT54YZ9I
|
NR1H3_HUMAN
|
Decreases Expression
|
[6] |
|
Nuclear factor 1 X-type (NFIX)
|
OT1DPZAE
|
NFIX_HUMAN
|
Decreases Expression
|
[6] |
|
Phosphoenolpyruvate carboxykinase , mitochondrial (PCK2)
|
OTJ8LX4N
|
PCKGM_HUMAN
|
Decreases Expression
|
[6] |
|
Organic solute transporter subunit alpha (SLC51A)
|
OTDJRZ0P
|
OSTA_HUMAN
|
Increases Expression
|
[6] |
|
Organic solute transporter subunit beta (SLC51B)
|
OT4WYPSR
|
OSTB_HUMAN
|
Increases Expression
|
[6] |
|
Liver-expressed antimicrobial peptide 2 (LEAP2)
|
OTUOPIS5
|
LEAP2_HUMAN
|
Affects Expression
|
[6] |
|
N-acetylmuramoyl-L-alanine amidase (PGLYRP2)
|
OTF8319A
|
PGRP2_HUMAN
|
Affects Expression
|
[6] |
|
Bile acid receptor (NR1H4)
|
OTWZLPTB
|
NR1H4_HUMAN
|
Decreases Expression
|
[6] |
|
Normal mucosa of esophagus-specific gene 1 protein (C15ORF48)
|
OT85J32K
|
NMES1_HUMAN
|
Affects Expression
|
[6] |
|
ATP-binding cassette sub-family G member 5 (ABCG5)
|
OT1OMY93
|
ABCG5_HUMAN
|
Affects Expression
|
[6] |
|
2-aminomuconic semialdehyde dehydrogenase (ALDH8A1)
|
OTINY3MW
|
AL8A1_HUMAN
|
Affects Expression
|
[6] |
|
Cytochrome P450 3A43 (CYP3A43)
|
OTMXADGA
|
CP343_HUMAN
|
Affects Expression
|
[6] |
|
NADPH oxidase 4 (NOX4)
|
OTTYQ097
|
NOX4_HUMAN
|
Decreases Expression
|
[6] |
|
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PPARGC1A)
|
OTHCDQ22
|
PRGC1_HUMAN
|
Decreases Expression
|
[6] |
|
Solute carrier family 22 member 7 (SLC22A7)
|
OTKTNH1W
|
S22A7_HUMAN
|
Affects Expression
|
[6] |
|
ATP-binding cassette sub-family C member 3 (ABCC3)
|
OTC3IJV4
|
MRP3_HUMAN
|
Decreases Expression
|
[14] |
|
ATP-dependent translocase ABCB1 (ABCB1)
|
OTEJROBO
|
MDR1_HUMAN
|
Decreases Expression
|
[14] |
|
Coxsackievirus and adenovirus receptor (CXADR)
|
OT9ZP02A
|
CXAR_HUMAN
|
Decreases Expression
|
[15] |
|
Hepatic sodium/bile acid cotransporter (SLC10A1)
|
OTUJVMCL
|
NTCP_HUMAN
|
Decreases Expression
|
[14] |
|
ATP-binding cassette sub-family C member 2 (ABCC2)
|
OTJSIGV5
|
MRP2_HUMAN
|
Decreases Expression
|
[14] |
| ------------------------------------------------------------------------------------ |
|
|
|
|