Details of the Drug
General Information of Drug (ID: DM17ONX)
| Drug Name |
Fexofenadine
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Carboxyterfenadine; Fexofendine; Terfenadine acid metabolite; Terfenadine carboxylate; F 9427; MDL 16455; Allegra (TN); Fastofen (TN); Fexofenadine (INN); Fexofenadine [INN:BAN]; Telfast (TN); Terfenadine-COOH; Terfenidine carboxylate, MDL 16455; Tilfur (TN); 2-[4-(1-hydroxy-4-{4-[hydroxy(diphenyl)methyl]piperidin-1-yl}butyl)phenyl]-2-methylpropanoic acid; 2-[4-[1-hydroxy-4-[4-[hydroxy(diphenyl)methyl]piperidin-1-yl]butyl]phenyl]-2-methylpropanoic acid; 4-(1-Hydroxy-4-(4-(hydroxydiphenylmethyl)-1-piperidinyl)butyl)-alpha,alpha-dimethylbenzeneacetic acid
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Antiallergic Agents
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
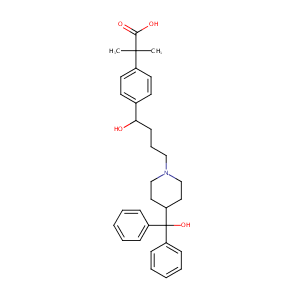 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 501.7 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 10 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Allergic rhinitis | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | CA08.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Fexofenadine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4819). | ||||
|---|---|---|---|---|---|
| 2 | Fexofenadine FDA Label | ||||
| 3 | DPD Approved Drugs: Allegra? oral tablets | ||||
| 4 | BDDCS applied to over 900 drugs | ||||
| 5 | Devillier P, Roche N, Faisy C: Clinical pharmacokinetics and pharmacodynamics of desloratadine, fexofenadine and levocetirizine : a comparative review. Clin Pharmacokinet. 2008;47(4):217-30. | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | Update on prescription and over-the-counter histamine inverse agonists in rhinitis therapy. Curr Allergy Asthma Rep. 2009 Mar;9(2):140-8. | ||||
| 8 | Involvement of multiple efflux transporters in hepatic disposition of fexofenadine. Mol Pharmacol. 2008 May;73(5):1474-83. | ||||
| 9 | Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther. 2007 Mar;81(3):362-70. | ||||
| 10 | The effects of the SLCO2B1 c.1457C>T polymorphism and apple juice on the pharmacokinetics of fexofenadine and midazolam in humans. Pharmacogenet Genomics. 2011 Feb;21(2):84-93. | ||||
| 11 | Influence of the flavonoids apigenin, kaempferol, and quercetin on the function of organic anion transporting polypeptides 1A2 and 2B1. Biochem Pharmacol. 2010 Dec 1;80(11):1746-53. | ||||
| 12 | Contribution of OATP (organic anion-transporting polypeptide) family transporters to the hepatic uptake of fexofenadine in humans. Drug Metab Dispos. 2005 Oct;33(10):1477-81. | ||||
| 13 | Effect of itraconazole on the pharmacokinetics and pharmacodynamics of fexofenadine in relation to the MDR1 genetic polymorphism. Clin Pharmacol Ther. 2005 Aug;78(2):191-201. | ||||
| 14 | An in vitro coculture system of human peripheral blood mononuclear cells with hepatocellular carcinoma-derived cells for predicting drug-induced liver injury. Arch Toxicol. 2021 Jan;95(1):149-168. doi: 10.1007/s00204-020-02882-4. Epub 2020 Aug 20. | ||||
| 15 | A variant 2677A allele of the MDR1 gene affects fexofenadine disposition. Clin Pharmacol Ther. 2004 Nov;76(5):418-27. doi: 10.1016/j.clpt.2004.08.002. | ||||
| 16 | Effect of histamine H1 receptor antagonists on TARC/CCL17 and MDC/CCL22 production from CD14+ cells induced by antigenic stimulation in vitro. Int Arch Allergy Immunol. 2011;155(1):38-51. doi: 10.1159/000318720. Epub 2010 Nov 25. | ||||
| 17 | The effect of fexofenadine on expression of intercellular adhesion molecule 1 and induction of apoptosis on peripheral eosinophils. Allergy Asthma Proc. 2005 Jul-Aug;26(4):292-8. | ||||
| 18 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 19 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 20 | Product Information. Multaq (dronedarone). sanofi-aventis , Bridgewater, NJ. | ||||
| 21 | Product Information. Allegra (fexofenadine). Hoechst Marion-Roussel Inc, Kansas City, MO. | ||||
| 22 | Product Information. Balversa (erdafitinib). Janssen Products, LP, Horsham, PA. | ||||
| 23 | Product Information. Tukysa (tucatinib). Seattle Genetics Inc, Bothell, WA. | ||||
| 24 | Product Information. Kalydeco (ivacaftor). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 25 | Product Information. Prevymis (letermovir). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 26 | Product Information. Harvoni (ledipasvir-sofosbuvir). Gilead Sciences, Foster City, CA. | ||||
| 27 | Bailey DG, Dresser GK, Munoz C, Freemar DJ, Kim RB "Reduction of fexofenadine bioavailability by fruit juices." Clin Pharmacol Ther 69 (2001): PI-82. [PMID: 15735611] | ||||
| 28 | Product Information. Rukobia (fostemsavir). ViiV Healthcare, Research Triangle Park, NC. | ||||
| 29 | Product Information. Nexletol (bempedoic acid). Esperion Therapeutics, Ann Arbor, MI. | ||||
| 30 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 31 | Product Information. Tabrecta (capmatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 32 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 33 | Product Information. Tasigna (nilotinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 34 | Product Information. Varubi (rolapitant). Tesaro Inc., Waltham, MA. | ||||
| 35 | Product Information. Zokinvy (lonafarnib). Eiger BioPharmaceuticals, Palo Alto, CA. | ||||
| 36 | Benoist G, van Oort I, et al "Drug-drug interaction potential in men treated with enzalutamide: Mind the gap." Br J Clin Pharmacol 0 (2017): epub. [PMID: 28881501] | ||||
| 37 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
| 38 | Allred AJ, Bowen CJ, Park JW, et al. "Eltrombopag increases plasma rosuvastatin exposure in healthy volunteers." Br J Clin Pharmacol 72 (2011): 321-9. [PMID: 21434975] | ||||
