Details of the Drug
General Information of Drug (ID: DMZSDGX)
| Drug Name |
Dicyclomine
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Atumin; Bentomine; Bentylol; Dicicloverina; Dicycloverin; Dicycloverine; Dicycloverinum; Dicymine; Diocyl; Dyspas; Formulex; Mamiesan; Merbentyl; Procyclomin; Sawamin; Spasmoban; Wyovin; Bentyl hydrochloride; Bentylol hydrochloride; Dicyclomine Hcl; Dicycloverin hydrochloride; Diethylaminocarbethoxybicyclohexyl hydrochloride; Diocyl hydrochloride; Kolantyl hydrochloride; Wyovin hydrochloride; GU8471000; Bentyl (TN); Bentylol (TN); Byclomine (TN); Di-syntramine; Dibent (TN); Dicicloverina [INN-Spanish]; Dicycloverine (INN); Dicycloverine [INN:BAN]; Dicycloverinum [INN-Latin]; Dicymine (TN); Dilomine (TN); Formulex (TN); Lomine (TN); Merbentyl (TN); Oxityl-P; Beta-Diethylaminoethyl 1-cyclohexylcyclohexanecarboxylate hydrochloride; Di-Spaz (TN); J.L. 998; Beta-Diethylaminoethyl-1-cyclohexylhexahydrobenzoate hydrochloride; Bicyclohexyl-1-carbonsaeure-2'diethylaminoethylester; [Bicyclohexyl]-1-carboxylic acid, 2-(diethylamino)ethyl ester hydrochloride; Cyclohexanecarboxylic acid, 1-cyclohexyl-, 2-(diethylamino)ethyl ester; [1,1'-Bicyclohexyl]-1-carboxylic acid, 2-(diethylamino)ethyl ester; (1,1'-Bicyclohexyl)-1-carboxylic acid, 2-(diethylamino)ethyl ester; (BICYCLOHEXYL)-1-CARBOXYLIC ACID, 2-(DIETHYLAMINO)ETHYL ESTER, HYDROCHLORIDE; (Bicyclohexyl)-1-carboxylic acid, 2-(diethylamino)ethyl ester; 1-Cyclohexylcyclohexanecarboxylic acid 2-(diethylamino)ethyl ester; 2-(diethylamino)ethyl 1,1'-bi(cyclohexyl)-1-carboxylate; 2-(diethylamino)ethyl 1-cyclohexylcyclohexane-1-carboxylate; 2-diethylaminoethyl 1-cyclohexylcyclohexane-1-carboxylate; 2-diethylaminoethyl 1-cyclohexylcyclohexane-1-carboxylate hydrochloride
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Anticholinergic Agents
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
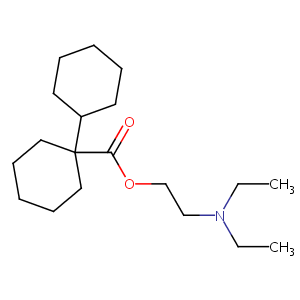 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 309.5 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.5 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Functional bowel syndrome | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | DD91.0 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Dicyclomine
Coadministration of a Drug Treating the Disease Different from Dicyclomine (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
|---|---|---|---|---|---|
| 2 | Dicyclomine FDA Label | ||||
| 3 | FDA Approved Drug Products: NORVIR (ritonavir) Capsules, Soft Gelatin for Oral use | ||||
| 4 | Metabolic dynamics of dicyclomine hydrochloride in man as influenced by various dose schedules and formulations Toxicology and Applied Pharmacology. 1967 Dec 18;13(1):16-23. | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | FDA Approved Drug Products: Dicyclomine Oral Capsules, Oral Tablets, and Intramuscular Injections | ||||
| 7 | [Characterization of muscarinic receptors in undifferentiated thyroid cells in Fisher rats]. Endocrinol Nutr. 2009 Mar;56(3):106-11. | ||||
| 8 | EMEA. European Medicines Agency "EPARs. European Union Public Assessment Reports.". | ||||
| 9 | Benjamin KW "Toxicity of ocular medications." Int Ophthalmol Clin 19 (1979): 199-255. [PMID: 376469] | ||||
| 10 | Product Information. Apadaz (acetaminophen-benzhydrocodone). KemPharm, Inc, Coralville, IA. | ||||
| 11 | Kulik AV, Wilbur R "Delirium and stereotypy from anticholinergic antiparkinson drugs." Prog Neuropsychopharmacol Biol Psychiatry 6 (1982): 75-82. [PMID: 7202232] | ||||
| 12 | Cohen MA, Alfonso CA, Mosquera M. Development of urinary retention during treatment with clozapine and meclizine [published correction appears in Am J Psychiatry 1994 Jun;151(6):952]. Am J Psychiatry. 1994;151(4):619-620. [PMID: 8147469] | ||||
| 13 | Beers MH, Ouslander JG, Rollingher I, Reuben DB, Brooks J, Beck JC "Explicit criteria for determining inappropriate medication use in nursing home residents." Arch Intern Med 151 (1991): 1825-32. [PMID: 1888249] | ||||
| 14 | Eronen M, Putkonen H, Hallikainen T, Vartiainen H "Lethal gastroenteritis associated with clozapine and loperamide." Am J Psychiatry 160 (2003): 2242-2243. [PMID: 14638602] | ||||
| 15 | Multum Information Services, Inc. Expert Review Panel. | ||||
| 16 | Cole JM, Sheehan AH, Jordan JK "Concomitant use of ipratropium and tiotropium in chronic obstructive plmonary disease." Ann Pharmacother 46 (2012): 1717-21. [PMID: 23170031] | ||||
| 17 | Product Information. Exalgo (hydromorphone). Covidien, Mansfield, MA. | ||||
| 18 | Product Information. GenESA (arbutamine). Gensia Inc, San Diego, CA. | ||||
| 19 | Linnoila M "Drug effects on psychomotor skills related to driving: interaction of atropine, glycopyrrhonium and alcohol." Eur J Clin Pharmacol 6 (1973): 107-12. [PMID: 4588850] | ||||
| 20 | Product Information. Zonegran (zonisamide) Elan Pharmaceuticals, S. San Francisco, CA. | ||||
| 21 | Product Information. Nizoral (ketoconazole). Janssen Pharmaceutica, Titusville, NJ. | ||||
| 22 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 23 | Beermann B, Groschinsky-Grind M "Enhancement of the gastrointestinal absorption of hydrochlorothiazide by propantheline." Eur J Clin Pharmacol 13 (1978): 385-7. [PMID: 668798] | ||||
| 24 | Postma JU, van Tilburg W "Visual hallucinations and delirium during treatment with amantadine (Symmetrel)." J Am Geriatr Soc 23 (1975): 212-5. [PMID: 123540] | ||||
| 25 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 26 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 27 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 28 | Product Information. Mestinon (pyridostigmine). ICN Pharmaceuticals Inc, Cost Mesa, CA. | ||||
| 29 | Product Information. Motilium (domperidone). Janssen-Ortho Inc, Toronto, ON. | ||||
| 30 | Algeri S, Cerletti C, Curcio M, et al. "Effect of anticholinergic drugs on gastro-intestinal absorption of L-dopa in rats and man." Eur J Pharmacol 35 (1976): 293-9. [PMID: 1248506] | ||||
| 31 | Product Information. Symlin (pramlintide). Amphastar Pharmaceuticals Inc, South El Monte, CA. | ||||
