Details of the Drug
General Information of Drug (ID: DMYWQ0O)
| Drug Name |
Tetrabenazine
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Nitoman; Rubigen; Tetrabenazina; Tetrabenazinum; Tetrabenzaine; Tetrabenzine; Xenazine; Tetra Benazin; Nitoman (TN); Ro 1-9569; Tetrabenazina [INN-Spanish]; Tetrabenazine (INN); Tetrabenazine [INN:BAN]; Tetrabenazinum [INN-Latin]; Xenazine (TN); Nitoman, Xenazine, Tetrabenazine; Ro 1-9569/12; 1,3,4,6,7,11b-Hexahydro-3-isobutyl-9,10-dimethoxy-2H-benzo(a)quinolizin-2-one; 1,3,4,6,7,11b-Hexahydro-3-isobutyl-9,10-dimethoxy-2H-benzo[a]quinolizin-2-one; 1,3,4,6,7,11b-hexahydro-9,10-dimethoxy-3-(2-methylpropyl)-2H-Benzo[a]quinolizin-2-one; 2-Oxo-3-isobutyl-9,10-dimethoxy-1,2,3,4,6,7-hexahydro-11bH-benzo[a]quinolizine; 2-Oxo-3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11-beta-hexahydro-2H-benzoquinolizine; 2-Oxo-3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11.beta.-hexahydro-2H-benzoquinolizine; 2H-Benzo(a)quinolizin-2-one, 1,3,4,6,7,11b-hexahydro-9,10-dimethoxy-3-(2-methylpropyl)-(9CI); 9,10-dimethoxy-3-(2-methylpropyl)-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-one; 9,10-dimethoxy-3-(2-methylpropyl)-1,3,4,6,7,11b-hexahydrobenzo[a]quinolizin-2-one
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Nephropathic cystinosis therapy
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
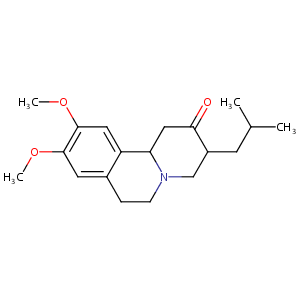 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 317.4 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.9 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Huntington disease | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 8A01.10 | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Tetrabenazine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4834). | ||||
|---|---|---|---|---|---|
| 2 | BDDCS applied to over 900 drugs | ||||
| 3 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 4 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 5 | Dopamine signaling is required for depolarization-induced slow current in cerebellar Purkinje cells. J Neurosci. 2009 Jul 1;29(26):8530-8. | ||||
| 6 | Role of tetrabenazine for Huntington's disease-associated chorea. Ann Pharmacother. 2010 Jun;44(6):1080-9. | ||||
| 7 | The effect of rare human sequence variants on the function of vesicular monoamine transporter 2. Pharmacogenetics. 2004 Sep;14(9):587-94. doi: 10.1097/00008571-200409000-00003. | ||||
| 8 | Product Information. Austedo (deutetrabenazine). Teva Pharmaceuticals USA, North Wales, PA. | ||||
| 9 | Product Information. Tibsovo (ivosidenib). Agios Pharmaceuticals, Cambridge, MA. | ||||
| 10 | Canadian Pharmacists Association. | ||||
| 11 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 12 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 13 | Product Information. Arcapta Neohaler (indacaterol). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 14 | Bengtsson B, Fagerstrom PO "Extrapulmonary effects of terbutaline during prolonged administration." Clin Pharmacol Ther 31 (1982): 726-32. [PMID: 7042176] | ||||
| 15 | Burke RE, Fahn S, Mayeux R, Weinberg H, Louis K, Willner JH "Neuroleptic malignant syndrome caused by dopamine-depleting drugs in a patient with Huntington disease." Neurology 31 (1981): 1022-5. [PMID: 6115336] | ||||
| 16 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 17 | Product Information. Ingrezza (valbenazine). Neurocrine Biosciences, Inc., San Diego, CA. | ||||
| 18 | EMEA. European Medicines Agency "EPARs. European Union Public Assessment Reports.". | ||||
| 19 | Product Information. Myrbetriq (mirabegron). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 20 | Product Information. Stribild (cobicistat/elvitegravir/emtricitabine/tenofov). Gilead Sciences, Foster City, CA. | ||||
| 21 | Product Information. Givlaari (givosiran). Alnylam Pharmaceuticals, Cambridge, MA. | ||||
| 22 | Product Information. Orladeyo (berotralstat). BioCryst Pharmaceuticals Inc, Durham, NC. | ||||
| 23 | Product Information. Xalkori (crizotinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 24 | Product Information. Xenazine (tetrabenazine). Prestwick Pharmaceuticals Inc, Washington DC, VA. | ||||
| 25 | Product Information. Tagrisso (osimertinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 26 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 27 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 28 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 29 | Product Information. Farydak (panobinostat). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 30 | Product Information. Varubi (rolapitant). Tesaro Inc., Waltham, MA. | ||||
| 31 | Product Information. Belviq (lorcaserin). Eisai Inc, Teaneck, NJ. | ||||
| 32 | Product Information. Macrilen (macimorelin). Aeterna Zentaris, Charleston, SC. | ||||
| 33 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
