Details of the Drug
General Information of Drug (ID: DMVPUQI)
| Drug Name |
Hydroflumethiazide
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Bristab; Bristurin; Dihydroflumethazide; Dihydroflumethiazide; Diucardin; Diuredemina; Diurometon; Elodrin; Elodrine; Enjit; Finuret; Flutizide; Glomerulin; HFZ; Hidroalogen; Hidroflumetiazid; Hidroflumetiazida; Hydol; Hydrenox; Hydroflumethazide; Hydroflumethiazidum; Hydroflumethizide; Idroflumetiazide; Leodrine; Metflorylthiazidine; Metforylthiadiazin; Metforylthiazidin; Methforylthiazidine; NaClex; Olmagran; Rivosil; Robezon; Rodiuran; Rontyl; Saluron; Sisuril; Spandiuril; Trifluoromethylhydrazide; Trifluoromethylhydrothiazide; Vergonil; Component of Salutensin; Idroflumetiazide [DCIT]; SB01887; Di-ademil; Di-adenil; Hidroflumetiazida [INN-Spanish]; Hydroflumethiazidum [INN-Latin]; Naciex (glaxo); Saluron (TN); Hydroflumethiazide [INN:BAN:JAN]; Hydroflumethiazide (JAN/USP/INN); Ethyl-2H-1,2, 4-benzothiadiazine-7-sulfonamide 1,1-dioxide; Fluoromethyl-3, 4-dihydro-1,2,4-benzothiadiazine 1,1-dioxide; 1,1-dioxo-6-(trifluoromethyl)-3,4-dihydro-2H-1; 2H-1,2, 4-Benzothiadiazine-7-sulfonamide, 3, 4-dihydro-6-(trifluoromethyl)-, 1,1-dioxide; 2H-1,2,4-Benzothiadiazine-7-sulfonamide, 3,4-dihydro-6-(trifluoromethyl)-, 1,1-dioxide; 3, 4-Dihydro-6-trifluorom; 3, 4-Dihydro-6-trifluoromethyl-7-sulfamoylbenzo-1,2,4-thiadiazine 1, 1-dioxide; 3,4-Dihydro-6-(trifluoromethyl)-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide; 3,4-Dihydro-6-trifluoromethyl-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide; 3,4-Dihydro-6-trifluoromethyl-7-sulfamoylbenzo-1,2,4-thiadiazine 1,1-dioxide; 3,4-Dihydro-7-sulfamoyl-6-trifluoromethyl-2H-1,2, 4-benzothi; 3,4-Dihydro-7-sulfamoyl-6-trifluoromethyl-2H-1,2,4-benzothiadiazine 1,1-dioxide; 3,4-Dihydro-7-sulfamyl-6-trifluoromethyl-2H-1,2,4-benzothiadiazine 1,1-dioxide; 6-(Trifluoromethyl)-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide; 6-Trifluoromethyl-3, 4-dihydro-7-sulfamoyl-2H-1,2,4-benzothiadiazine 1,1-dioxide; 6-Trifluoromethyl-3,4-dihydro-7-sulfamoyl-2H-1,2,4-benzothiadiazine 1,1-dioxide; 6-Trifluoromethyl-7-sulfamoyl-3,4-dihydro-1,2, 4-benzothiadiazine-1,1-dioxide; 6-Trifluoromethyl-7-sulfamoyl-3,4-dihydro-1,2,4-benzothiadiazine-1,1-dioxide; 6-Trifluoromethyl-7-sulfamyl-3,4-dihydro-1,2,4-benzothiadiazine-1,1-dioxide; 7-Sulfamoyl-6-tri; 7-Sulfamoyl-6-trifluoromethyl-3,4-dihydro-1,2,4-benzothiadiazine 1,1-dioxide; 7-Sulfamyl-6-trifluoromethyl-3,4-dihydro-1,2,4-benzothiadiazine 1, 1-dioxide; 7-Sulfamyl-6-trifluoromethyl-3,4-dihydro-1,2,4-benzothiadiazine 1,1-dioxide
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Diuretics
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
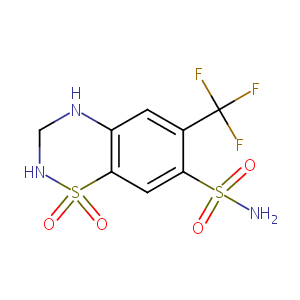 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 331.3 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.4 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 10 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Hydroflumethiazide
Coadministration of a Drug Treating the Disease Different from Hydroflumethiazide (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
