Details of the Drug
General Information of Drug (ID: DM60QMR)
| Drug Name |
Ethacrynic acid
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Crinuryl; Edecril; Edecrina; Endecril; Ethacrynate; Hidromedin; Hydromedin; Mingit; Otacril; Reomax; Taladren; Uregit; Acide etacrynique; Acido etacrinico; Acidum etacrynicum; Etacrinic acid; Etacrynic Acid; Etakrinic acid; Ethacrinic acid; Ethacryinic Acid; Kyselina ethakrynova; Kyselina ethakrynova [Czech]; Methylenebutyryl phenoxyacetic acid; Methylenebutyrylphenoxyacetic acid; E0526; MK 595; Acide etacrynique [INN-French]; Acido etacrinico [INN-Spanish]; Acidum etacrynicum [INN-Latin]; Edecrin (TN); Ethacrinique (acide); Ethacrynic Acid, Sodium Salt; Ethacrynic acid (USP); Ethacrynic acid [USAN:BAN]; MK-595; Etacrynic acid (JP15/INN); [2,3-Dichloro-4-(2-methylenebutyryl)phenoxy]acetic acid; [2,3-dichloro-4-(2-methylenebutanoyl)phenoxy]acetic acid; [2,3-dichloro-4-(2-methylidenebutanoyl)phenoxy]acetic acid; [4-(2-Methylenebutyryl)-2,3-dichlorophenoxy]acetic acid; Kyselina 4-(2-(1-butenyl)karbonyl)-2,3-dichlorfenoxyoctova; Kyselina 4-(2-(1-butenyl)karbonyl)-2,3-dichlorfenoxyoctova [Czech]; [2,3-Dichloro-4-(2-methylene-1-oxobutyl)phenoxy]acetic acid; (2,3-Dichloro-4-(2-methylene-1-oxobutyl)phenoxy)acetic acid; (2,3-Dichloro-4-(2-methylenebutyryl)phenoxy)acetic acid; (2,3-Dichloro-4-[2-methylenebutyryl]phenoxy)acetic acid; (4-(2-Methylenebutyryl)-2,3-dichlorophenoxy)acetic acid; 2,3-Dichloro-4(2-methylene-butyryl)phenoxy] acetic acid; 2,3-Dichloro-4-(2-methylenebutyl)phenoxyacetic acid; 2,3-Dichloro-4-(2-methylenebutyryl)phenoxy acetic acid; 2,3-Dichloro-4-(2-methylenebutyryl)phenoxyacetic acid; 2-[2,3-dichloro-4-(2-methylidenebutanoyl)phenoxy]acetic acid
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Diuretics
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
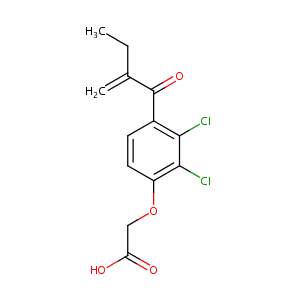 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 303.13 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.8 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Ethacrynic acid (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Ethacrynic acid FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7179). | ||||
| 3 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 4 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | Na-K-Cl cotransport regulates intracellular volume and monolayer permeability of trabecular meshwork cells. Am J Physiol. 1995 Apr;268(4 Pt 1):C1067-74. | ||||
| 7 | Inhibition of the multidrug resistance protein 1 (MRP1) by peptidomimetic glutathione-conjugate analogs. Mol Pharmacol. 2002 Nov;62(5):1160-6. | ||||
| 8 | Xenobiotic metabolizing and antioxidant enzymes in normal and neoplastic human breast tissue. Eur J Drug Metab Pharmacokinet. 1998 Oct-Dec;23(4):497-500. | ||||
| 9 | Purification and biochemical properties of glutathione S-transferase from Lactuca sativa. J Biochem Mol Biol. 2005 Mar 31;38(2):232-7. | ||||
| 10 | Isoform-specific induction of a human aldo-keto reductase by polycyclic aromatic hydrocarbons (PAHs), electrophiles, and oxidative stress: implications for the alternative pathway of PAH activation catalyzed by human dihydrodiol dehydrogenase. Cancer Res. 1999 Feb 1;59(3):607-14. | ||||
| 11 | Effects of fibrates on human organic anion-transporting polypeptide 1B1-, multidrug resistance protein 2- and P-glycoprotein-mediated transport. Xenobiotica. 2005 Jul;35(7):737-53. | ||||
| 12 | Ethacrynic acid and a derivative enhance apoptosis in arsenic trioxide-treated myeloid leukemia and lymphoma cells: the role of glutathione S-transferase p1-1. Clin Cancer Res. 2012 Dec 15;18(24):6690-701. doi: 10.1158/1078-0432.CCR-12-0770. Epub 2012 Oct 18. | ||||
| 13 | Genotoxic effect of ethacrynic acid and impact of antioxidants. Toxicol Appl Pharmacol. 2015 Jul 1;286(1):17-26. doi: 10.1016/j.taap.2015.03.016. Epub 2015 Mar 25. | ||||
| 14 | Curcumin-induced GADD153 upregulation: modulation by glutathione. J Cell Biochem. 2007 May 15;101(2):307-20. doi: 10.1002/jcb.21179. | ||||
| 15 | Chrysos G, Gargalianos P, Lelekis M, Stefanou J, Kosmidis J "Pharmacokinetic interactions of ceftazidime and frusemide." J Chemother 7 Suppl (1995): 107-10. [PMID: 8904125] | ||||
| 16 | Product Information. Multaq (dronedarone). sanofi-aventis , Bridgewater, NJ. | ||||
| 17 | Bates DE, Beaumont SJ, Baylis BW "Ototoxicity induced by gentamicin and furosemide." Ann Pharmacother 36 (2002): 446-51. [PMID: 11895059] | ||||
| 18 | Product Information. Nexium (esomeprazole) Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 19 | Product Information. Clozaril (clozapine). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 20 | Product Information. Skelid (tilundronate). Sanofi Winthrop Pharmaceuticals, New York, NY. | ||||
| 21 | Muller FO, Schall R, Devaal AC, Groenewoud G, Hundt HKL, Middle MV "Influence of meloxicam on furosemide pharmacokinetics and pharmacodynamics in healthy volunteers." Eur J Clin Pharmacol 48 (1995): 247-51. [PMID: 7589049] | ||||
| 22 | Product Information. Savella (milnacipran). Forest Pharmaceuticals, St. Louis, MO. | ||||
| 23 | Product Information. Marplan (isocarboxazid) Roche Laboratories, Nutley, NJ. | ||||
| 24 | Product Information. Aptiom (eslicarbazepine). Sunovion Pharmaceuticals Inc, Marlborough, MA. | ||||
| 25 | Dean S, Kendall MJ, Potter S, Thompson MH, Jackson DA "Nadolol in combination with indapamide and xipamide in resistant hypertensives." Eur J Clin Pharmacol 28 (1985): 29-33. [PMID: 3987783] | ||||
| 26 | Semple P, Tilstone WJ, Lawson DH "Furosemide and urinary digoxin clearance." N Engl J Med 293 (1975): 612-3. [PMID: 902451] | ||||
| 27 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 28 | Murphy BF, Whitworth JA, Kincaid-Smith P "Renal insufficiency with combinations of angiotensin converting enzyme inhibitors and diuretics." Br Med J 288 (1984): 844-5. [PMID: 6322905] | ||||
| 29 | Leary WP, Reyes AJ "Drug interactions with diuretics." S Afr Med J 65 (1984): 455-61. [PMID: 6701709] | ||||
| 30 | Ohnishi K, Yoshida H, Shigeno K, et al. "Prolongation of the QT interval and ventricular tachycardia in patients treated with arsenic trioxide for acute promyelocytic leukemia." Ann Intern Med 133 (2000): 881-5. [PMID: 11103058] | ||||
| 31 | Cohen J "Long-term efficacy and safety of terazosin alone and in combination with other antihypertensive agents." Am Heart J 122 (1991): 919-25. [PMID: 1678923] | ||||
| 32 | Langer T, am Zehnhoff-Dinnesen A, Radtke S, Meitert J, Zolk O "Understanding platinum-induced ototoxicity." Trends Pharmacol Sci 34 (2013): 458-69. [PMID: 23769626] | ||||
| 33 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 34 | Dresser GK, Spence JD, Bailey DG "Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition." Clin Pharmacokinet 38 (2000): 41-57. [PMID: 10668858] | ||||
| 35 | Antonelli D, Atar S, Freedberg NA, Rosenfeld T "Torsade de pointes in patients on chronic amiodarone treatment: contributing factors and drug interactions." Isr Med Assoc J 7 (2005): 163-5. [PMID: 15792261] | ||||
