Details of the Drug
General Information of Drug (ID: DMA6S1D)
| Drug Name |
Ropinirole
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Ropinirol; Ropinirolum; Ropitor; ReQuip CR; ReQuip XL; SKF 101468; Adartrel (TN); Requip (TN); Ropark (TN); Ropinirol[INN-Spanish]; Ropinirole (INN); Ropinirole [INN:BAN]; Ropinirolum [INN-Latin]; Ropitor (TN); SK&F 101468; SK&F-101,468; 4-[2-(Dipropylamino)ethyl]-1,3-dihydro-2H-indol-2-one; 4-[2-(Dipropylamino)ethyl]indoline-2-one; 4-[2-(dipropylamino)ethyl]-1,3-dihydro-2H-indol-2-one; 4-[2-(dipropylamino)ethyl]-1,3-dihydroindol-2-one
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antiparkinson Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
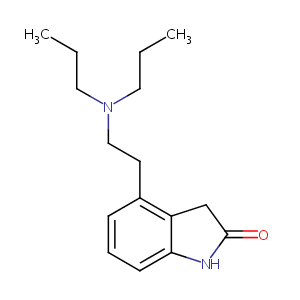 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 260.37 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Parkinson disease | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 8A00.0 | |||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Ropinirole (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7295). | ||||
|---|---|---|---|---|---|
| 2 | FDA Approved Drug Products: OZEMPIC (semaglutide) injection, for subcutaneous use | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Ahire D, Sinha S, Brock B, Iyer R, Mandlekar S, Subramanian M: Metabolite Identification, Reaction Phenotyping, and Retrospective Drug-Drug Interaction Predictions of 17-Deacetylnorgestimate, the Active Component of the Oral Contraceptive Norgestimate. Drug Metab Dispos. 2017 Jun;45(6):676-685. doi: 10.1124/dmd.116.073940. Epub 2017 Mar 10. | ||||
| 5 | Clinical pharmacokinetics of ropinirole. Clin Pharmacokinet. 2000 Oct;39(4):243-54. doi: 10.2165/00003088-200039040-00001. | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 8 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | ||||
| 9 | In vitro identification of the P450 enzymes responsible for the metabolism of ropinirole. Drug Metab Dispos. 1997 Jul;25(7):840-4. | ||||
| 10 | Receptor-binding and pharmacokinetic properties of dopaminergic agonists. Curr Top Med Chem. 2008;8(12):1049-67. | ||||
| 11 | D2/D3 receptor agonist ropinirole protects dopaminergic cell line against rotenone-induced apoptosis through inhibition of caspase- and JNK-dependent pathways. FEBS Lett. 2008 Mar 5;582(5):603-10. doi: 10.1016/j.febslet.2008.01.028. Epub 2008 Jan 31. | ||||
| 12 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 13 | Mims RB, Scott CL, Modebe O, Bethune JE "Inhibition of L-dopa-induced growth hormone stimulation by pyridoxine and chlorpromazine." J Clin Endocrinol Metab 40 (1975): 256-9. [PMID: 1117978] | ||||
| 14 | Product Information. Noroxin (norfloxacin). Merck & Co, Inc, West Point, PA. | ||||
| 15 | Warrington SJ, Ankier SI, Turner P "Evaluation of possible interactions between ethanol and trazodone or amitriptyline." Neuropsychobiology 15 (1986): 31-7. [PMID: 3725002] | ||||
| 16 | Product Information. Naropin (ropivacaine). Astra USA, Westborough, MA. | ||||
| 17 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 18 | Product Information. Alphagan (brimonidine ophthalmic). Allergan Inc, Irvine, CA. | ||||
| 19 | Product Information. Tagrisso (osimertinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 20 | Product Information. Zelboraf (vemurafenib). Genentech, South San Francisco, CA. | ||||
| 21 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 22 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 23 | Product Information. Exjade (deferasirox). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 24 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 25 | Product Information. Thalomid (thalidomide). Celgene Corporation, Warren, NJ. | ||||
| 26 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 27 | Hansen BS, Dam M, Brandt J, et al "Influence of dextropropoxyphene on steady state serum levels and protein binding of three anti-epileptic drugs in man." Acta Neurol Scand 61 (1980): 357-67. [PMID: 6998251] | ||||
| 28 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 29 | Bair JD, Oppelt TF "Warfarin and ropinirole interaction." Ann Pharmacother 35 (2001): 1202-4. [PMID: 11675845] | ||||
| 30 | Product Information. Zanaflex (tizanidine). Acorda Therapeutics, Hawthorne, NY. | ||||
| 31 | Product Information. Norprolac (quinagolide). Ferring Inc, North York, IA. | ||||
| 32 | Product Information. Zyrtec (cetirizine). Pfizer US Pharmaceuticals, New York, NY. | ||||
