Details of the Drug
General Information of Drug (ID: DMCTE9R)
| Drug Name |
Mexiletine
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Mexiletene; Mexiletina; Mexiletinum; Mexilitine; Mexityl; Mexiletine HCL; KO1173; KO-1173; KOE-1173; Mexiletina [INN-Spanish]; Mexiletine (INN); Mexiletine [INN:BAN]; Mexiletinum [INN-Latin]; Mexitil (TN); (+-)-1-(2,6-dimethylphenoxy)propan-2-amine; (2RS)-1-(2,6-dimethylphenoxy)-2-aminopropane; 1-(2',6'-Dimethylphenoxy)-2-aminopropane; 1-(2,6-Dimethylphenoxy)-2-propanamine; 1-(2,6-dimethylphenoxy)propan-2-amine; 1-Methyl-2-(2,6-xylyloxy)ethylamine; 1-methyl-2-(2,6-xylyloxy)ethanamine; 2-(2-Aminopropoxy)-1,3-DiMethyl-Benzene Hydrochloride; 2-(2-aminopropoxy)-1,3-dimethylbenzene
|
|||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||
| Therapeutic Class |
Antiarrhythmic Agents
|
|||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||
| Structure |
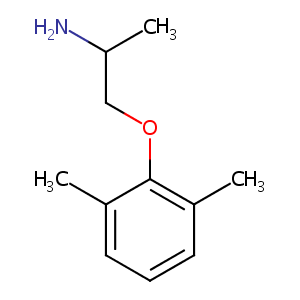 |
|||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 179.26 | ||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.1 | |||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | |||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | |||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | |||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Mexiletine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
|---|---|---|---|---|---|
| 2 | BDDCS applied to over 900 drugs | ||||
| 3 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 4 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 7 | Effect of sodium channel blockers on ST segment, QRS duration, and corrected QT interval in patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2000 Dec;11(12):1320-9. | ||||
| 8 | Pharmacokinetic and pharmacodynamic interaction between mexiletine and propafenone in human beings. Clin Pharmacol Ther. 2000 Jul;68(1):44-57. | ||||
| 9 | Role of specific cytochrome P450 enzymes in the N-oxidation of the antiarrhythmic agent mexiletine. Xenobiotica. 2003 Jan;33(1):13-25. | ||||
| 10 | Impact of CYP2D6*10 on mexiletine pharmacokinetics in healthy adult volunteers. Eur J Clin Pharmacol. 2003 Sep;59(5-6):395-9. | ||||
| 11 | Inhibition of human liver cytochrome P-450 1A2 by the class IB antiarrhythmics mexiletine, lidocaine, and tocainide. J Pharmacol Exp Ther. 1999 May;289(2):853-8. | ||||
| 12 | Mitchell BG, Clements JA, Pottage A, Prescott LF "Mexiletine disposition: individual variation in response to urine acidification and alkalinisation." Br J Clin Pharmacol 16 (1983): 281-4. [PMID: 6626420] | ||||
| 13 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 14 | Product Information. Multaq (dronedarone). sanofi-aventis , Bridgewater, NJ. | ||||
| 15 | Hurwitz A, Vacek JL, Botteron GW, et al "Mexiletine effects on theophylline disposition." Clin Pharmacol Ther 50 (1991): 299-307. [PMID: 1914365] | ||||
| 16 | Product Information. Ocaliva (obeticholic acid). Intercept Pharmaceuticals, Inc., New York, NY. | ||||
| 17 | Granfors MT, Backman JT, Neuvonen M, Neuvonen PJ. Ciprofloxacin greatly increases concentrations and hypotensive effect of tizanidine by inhibiting its cytochrome P450 1A2-mediated presystemic metabolism.?Clin Pharmacol Ther. 2004;76(6):598-606. [PMID: 15592331] | ||||
| 18 | Alfaro CL, Lam YWF, Simpson J, Ereshefsky L "CYP2D6 status of extensive metabolizers after multiple-dose fluoxetine, fluvoxamine, paroxetine, or sertraline." J Clin Psychopharmacol 19 (1999): 155-63. [PMID: 10211917] | ||||
| 19 | Product Information. Cymbalta (duloxetine). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 20 | Magnusson M, Bergstrand IC, Bjorkman S, Heijl A, Roth B, Hoglund P "A placebo-controlled study of retinal blood flow changes by pentoxifylline and metabolites in humans." Br J Clin Pharmacol 61 (2006): 138-47. [PMID: 16433868] | ||||
| 21 | Begg EJ, Chinwah PM, Webb C, Day RO, Wade DN "Enhanced metabolism of mexiletine after phenytoin administration." Br J Clin Pharmacol 14 (1982): 219-23. [PMID: 7104173] | ||||
| 22 | Product Information. Myrbetriq (mirabegron). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 23 | Product Information. Enablex (darifenacin). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 24 | AbdelRahman SM, Gotschall RR, Kauffman RE, Leeder JS, Kearns GL "Investigation of terbinafine as a CYP2D6 inhibitor in vivo." Clin Pharmacol Ther 65 (1999): 465-72. [PMID: 10340911] | ||||
| 25 | Product Information. Olysio (simeprevir). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 26 | Pentikainen PJ, Koivula IH, Hiltunen HA "Effect of rifampicin treatment on the kinetics of mexiletine." Eur J Clin Pharmacol 23 (1982): 261-6. [PMID: 6129140] | ||||
| 27 | Product Information. Intelence (etravirine). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 28 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 29 | Product Information. Givlaari (givosiran). Alnylam Pharmaceuticals, Cambridge, MA. | ||||
| 30 | Product Information. Orladeyo (berotralstat). BioCryst Pharmaceuticals Inc, Durham, NC. | ||||
| 31 | Product Information. Rozerem (ramelteon). Takeda Pharmaceuticals America, Lincolnshire, IL. | ||||
| 32 | Product Information. Hetlioz (tasimelteon). Vanda Pharmaceuticals Inc, Rockville, MD. | ||||
| 33 | Product Information. Suprep Bowel Prep Kit (magnesium/potassium/sodium sulfates). Braintree Laboratories, Braintree, MA. | ||||
| 34 | Product Information. Lotronex (alosetron). Glaxo Wellcome, Research Triangle Park, NC. | ||||
| 35 | Product Information. Vizimpro (dacomitinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 36 | Product Information. Zelboraf (vemurafenib). Genentech, South San Francisco, CA. | ||||
| 37 | Product Information. Exjade (deferasirox). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 38 | Product Information. Farydak (panobinostat). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 39 | Product Information. Gleevec (imatinib mesylate). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 40 | Canadian Pharmacists Association. | ||||
| 41 | Product Information. Varubi (rolapitant). Tesaro Inc., Waltham, MA. | ||||
| 42 | Product Information. Wellbutrin XL (buPROPion). GlaxoSmithKline, Philadelphia, PA. | ||||
| 43 | Product Information. Belviq (lorcaserin). Eisai Inc, Teaneck, NJ. | ||||
| 44 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 45 | Matsuoka LY "Convulsions following application of gamma benzene hexachloride." J Am Acad Dermatol 5 (1981): 98-9. [PMID: 6168673] | ||||
| 46 | Product Information. Zytiga (abiraterone). Centocor Inc, Malvern, PA. | ||||
| 47 | Product Information. Celebrex (celecoxib). Searle, Chicago, IL. | ||||
| 48 | Product Information. Saphris (asenapine). Schering-Plough Corporation, Kenilworth, NJ. | ||||
| 49 | Granfors MT, Backman JT, Laitila J, Neuvonen PJ "Tizanidine is mainly metabolized by cytochrome P450 1A2 in vitro." Br J Clin Pharmacol 57 (2004): 349-53. [PMID: 14998432] | ||||
