Details of the Drug
General Information of Drug (ID: DMYTE6L)
| Drug Name |
Enoxacin
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Almitil; Bactidan; Comprecin; Enoram; Enoxacine; Enoxacino; Enoxacinum; Enoxin; Enoxor; Flumark; Penetrex; Enoxacin Sesquihydrate; Enoxacine [French]; Enoxacino [Spanish]; Enoxacinum [Latin]; Faulding Brand of Enoxacin; Pierre Fabre Brand of Enoxacin Sesquihydrate; Rhone Poulenc Rorer Brand of Enoxacin Sesquihydrate; AT 2266; AT2266; CI919; CL23362; E0762; PD 107779; PD107779; AT-2266; Almitil (TN); Bactidan (TN); Bactidron (TN); Comprecin (TN); Enoksetin (TN); Enoxen (TN); Enoxin (TN); Enoxor (TN); Enroxil (TN); Flumark (TN); Gyramid (TN); PD-107779; Penetrex (TN); Rhone-Poulenc Rorer Brand of Enoxacin Sesquihydrate; Sesquihydrate, Enoxacin; Vinone (TN); Enoxacin (USAN/INN); Enoxacin [USAN:BAN:INN:JAN]; 1,8-Naphthyridine-3-carboxylic acid, 6-fluoro-1,4-dihydro-4-oxo-7-piperazinyl; 1-Ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-1,8-naphthyridine-3-carboxylic acid; 1-Ethyl-6-fluoro-1,4-dihydro-4-oxo-7-[1-piperazinyl]-1,8-naphthyridine-3-carboxylic acid; 1-Ethyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid; 1-ethyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid; 1-ethyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid; 1-ethyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,8-naphthyridine-3-carboxylic acid
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Antiinfective Agents
|
||||||||||||||||||||||||||
| Affected Organisms |
Enteric bacteria and other eubacteria
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
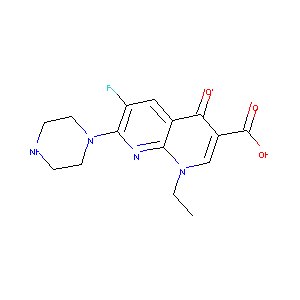 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 320.32 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.2 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Enoxacin (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Enoxacin FDA Label | ||||
|---|---|---|---|---|---|
| 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 019616. | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | Clinical pharmacokinetics of the newer antibacterial 4-quinolones. Clin Pharmacokinet. 1988 Feb;14(2):96-121. | ||||
| 7 | Identification of influx transporter for the quinolone antibacterial agent levofloxacin. Mol Pharm. 2007 Jan-Feb;4(1):85-94. | ||||
| 8 | Functional characterization of multidrug and toxin extrusion protein 1 as a facilitative transporter for fluoroquinolones. J Pharmacol Exp Ther. 2009 Feb;328(2):628-34. | ||||
| 9 | Interaction study between enoxacin and fluvoxamine. Ther Drug Monit. 2005 Jun;27(3):349-53. | ||||
| 10 | Computational and experimental studies on the inhibitory mechanism of hydroxychloroquine on hERG. Toxicology. 2021 Jun 30;458:152822. doi: 10.1016/j.tox.2021.152822. Epub 2021 May 28. | ||||
| 11 | Johnson EJ, MacGowan AP, Potter MN, et al "Reduced absorption of oral ciprofloxacin after chemotherapy for haematological malignancy." J Antimicrob Chemother 25 (1990): 837-42. [PMID: 2373666] | ||||
| 12 | Akerele JO, Okhamafe AO "Influence of oral co-administered metallic drugs on ofloxacin pharmacokinetics." J Antimicrob Chemother 28 (1991): 87-94. [PMID: 1663108] | ||||
| 13 | Beckmann J, Elsaber W, Gundert-Remy U, Hertrampf R "Enoxacin: a potent inhibitor of theophylline metabolism." Eur J Clin Pharmacol 33 (1987): 227-30. [PMID: 3480222] | ||||
| 14 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 15 | Product Information. Factive (gemifloxacin). GeneSoft Inc, San Francisco, CA. | ||||
| 16 | Friedman CI, Huneke AL, Kim MH, Powell J "The effect of ampicillin on oral contraceptive effectiveness." Obstet Gynecol 55 (1980): 33-7. [PMID: 7188714] | ||||
| 17 | Product Information. CellCept (mycophenolate mofetil). Roche Laboratories, Nutley, NJ. | ||||
| 18 | Product Information. Cymbalta (duloxetine). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 19 | Magnusson M, Bergstrand IC, Bjorkman S, Heijl A, Roth B, Hoglund P "A placebo-controlled study of retinal blood flow changes by pentoxifylline and metabolites in humans." Br J Clin Pharmacol 61 (2006): 138-47. [PMID: 16433868] | ||||
| 20 | Lebsack ME, Nix D, Ryerson B, et al "Effect of gastric acidity on enoxacin absorption." Clin Pharmacol Ther 52 (1992): 252-6. [PMID: 1526081] | ||||
| 21 | Deppermann KM, Lode H "Fluoroquinolones: interaction profile during enteral absorption." Drugs 45 Suppl 3 (1993): 65-72. [PMID: 7689454] | ||||
| 22 | Product Information. Nexletol (bempedoic acid). Esperion Therapeutics, Ann Arbor, MI. | ||||
| 23 | FDA. U.S. Food and Drug Administration "Information for Healthcare Professionals. Fluoroquinolone Antimicrobial Drugs. FDA Alert [7/8/2008].". | ||||
| 24 | Davey PG "Overview of drug interactions with the quinolones." J Antimicrob Chemother 22(suppl c) (1988): 97-107. [PMID: 3053579] | ||||
| 25 | Product Information. Rozerem (ramelteon). Takeda Pharmaceuticals America, Lincolnshire, IL. | ||||
| 26 | Product Information. Hetlioz (tasimelteon). Vanda Pharmaceuticals Inc, Rockville, MD. | ||||
| 27 | Product Information. Suprep Bowel Prep Kit (magnesium/potassium/sodium sulfates). Braintree Laboratories, Braintree, MA. | ||||
| 28 | Product Information. Lotronex (alosetron). Glaxo Wellcome, Research Triangle Park, NC. | ||||
| 29 | Product Information. Viberzi (eluxadoline). Actavis Pharma, Inc., Parsippany, NJ. | ||||
| 30 | Canadian Pharmacists Association. | ||||
| 31 | Product Information. Rilutek (riluzole). Rhone-Poulenc Rorer, Collegeville, PA. | ||||
| 32 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 33 | Product Information. Wellbutrin XL (buPROPion). GlaxoSmithKline, Philadelphia, PA. | ||||
| 34 | Product Information. Saphris (asenapine). Schering-Plough Corporation, Kenilworth, NJ. | ||||
| 35 | Krahenbuhl S, Sauter B, Kupferschmidt H, Krause M, Wyss PA, Meier PJ "Case report: reversible QT prolongation with torsades de pointes in a patient with pimozide intoxication." Am J Med Sci 309 (1995): 315-6. [PMID: 7771501] | ||||
| 36 | Granfors MT, Backman JT, Laitila J, Neuvonen PJ "Tizanidine is mainly metabolized by cytochrome P450 1A2 in vitro." Br J Clin Pharmacol 57 (2004): 349-53. [PMID: 14998432] | ||||
