Details of the Drug
General Information of Drug (ID: DMWOSKJ)
| Drug Name |
Zolpidem
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
zolpidem; 82626-48-0; Ambien; Zolpidemum; Lorex; Zolpidemum [Latin]; Zolpidem [INN:BAN]; UNII-7K383OQI23; N,N-Dimethyl-2-[6-methyl-2-(4-methylphenyl)imidazo[1,2-a]pyridin-3-yl]acetamide; CHEMBL911; N,N,6-Trimethyl-2-(4-methylphenyl)imidazo(1,2-a)pyridine-3-acetamide; DEA No 2783; CHEBI:10125; ZAFYATHCZYHLPB-UHFFFAOYSA-N; 7K383OQI23; NCGC00095179-01; N,N,6-Trimethyl-2-p-tolylimidazo[1,2-a]pyridine-3-acetamide; SL-800750; N,N,6-Trimethyl-2-(4-methylphenyl)imidazo[1,2-a]pyridine-3-acetamide; ZolpiMist; Zolpidem tartrate; Zolpidem (low-dose oral spray, middle-of-the-night awakenings); Zolpidem (low-dose oral spray, middle-of-the-night awakenings), NovaDel; [3H]zolpidem
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
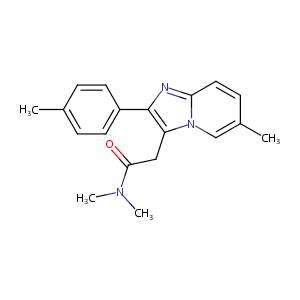 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 307.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Zolpidem (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4348). | ||||
|---|---|---|---|---|---|
| 2 | BDDCS applied to over 900 drugs | ||||
| 3 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 4 | LaCagnin LB, Lutsie P, Colby HD: Conversion of spironolactone to 7 alpha-thiomethylspironolactone by hepatic and renal microsomes. Biochem Pharmacol. 1987 Oct 15;36(20):3439-44. | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 7 | Zolpidem, a selective GABA(A) receptor alpha1 subunit agonist, induces comparable Fos expression in oxytocinergic neurons of the hypothalamic paraventricular and accessory but not supraoptic nuclei in the rat.Brain Res Bull.2006 Dec 11;71(1-3):200-7. | ||||
| 8 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 415). | ||||
| 9 | Zolpidem pharmacokinetics and pharmacodynamics in metabolic interactions involving CYP3A: sex as a differentiating factor. Eur J Clin Pharmacol. 2010 Sep;66(9):955. | ||||
| 10 | Effect of zolpidem on human cytochrome P450 activity, and on transport mediated by P-glycoprotein. Biopharm Drug Dispos. 2002 Dec;23(9):361-7. | ||||
| 11 | The influences of CYP2C9*1/*3 genotype on the pharmacokinetics of zolpidem. Arch Pharm Res. 2018 Sep;41(9):931-936. | ||||
| 12 | Zolpidem extended-release: a single insomnia treatment option for sleep induction and sleep maintenance symptoms. Am J Ther. 2007 May-Jun;14(3):299-305. | ||||
| 13 | Adverse reactions to zolpidem: case reports and a review of the literature. Prim Care Companion J Clin Psychiatry. 2010;12(6). pii: PCC.09r00849. | ||||
| 14 | PharmGKB: A worldwide resource for pharmacogenomic information. Wiley Interdiscip Rev Syst Biol Med. 2018 Jul;10(4):e1417. (ID: PA166170041) | ||||
| 15 | Principles of Clinical Pharmacology (Third Edition). 2012, Pages 417-436 | ||||
| 16 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 17 | US Food and Drug Administration "FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines requires its strongest warning.". | ||||
| 18 | Product Information. Multaq (dronedarone). sanofi-aventis , Bridgewater, NJ. | ||||
| 19 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 20 | Farkas D, Volak L, Harmatz J, von Moltke L, Court M, Greenblatt D "Short-term clarithromycin administration impairs clearance and enhances pharmacodynamic effects of trazodone but not of zolpidem." Clin Pharmacol Ther 85 (2009): 644-50. [PMID: 19242403] | ||||
| 21 | Greenblatt DJ, vonMoltke LL, Harmatz JS, Mertzanis P, Graf JA, Durol ALB, Counihan M, RothSchechter B, Shader RI "Kinetic and dynamic interaction study of zolpidem with ketoconazole, itraconazole, and fluconazole." Clin Pharmacol Ther 64 (1998): 661-71. [PMID: 9871431] | ||||
| 22 | Product Information. Ketek (telithromycin). Aventis Pharmaceuticals, Bridgewater, NJ. | ||||
| 23 | Product Information. Tykerb (lapatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 24 | Product Information. Piqray (alpelisib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 25 | US Food and Drug Administration "FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines requires its strongest warning.". | ||||
| 26 | Warrington SJ, Ankier SI, Turner P "Evaluation of possible interactions between ethanol and trazodone or amitriptyline." Neuropsychobiology 15 (1986): 31-7. [PMID: 3725002] | ||||
| 27 | Gill SS, Wright EM, Reilly CS "Pharmacokinetic interaction of propofol and fentanyl: single bolus injection study." Br J Anaesth 65 (1990): 760-5. [PMID: 2265045] | ||||
| 28 | Product Information. Kalydeco (ivacaftor). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 29 | Product Information. Prevymis (letermovir). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 30 | Product Information. Emend (aprepitant). Merck & Company Inc, West Point, PA. | ||||
| 31 | Product Information. Trileptal (oxcarbazepine) Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 32 | Product Information. Alphagan (brimonidine ophthalmic). Allergan Inc, Irvine, CA. | ||||
| 33 | Product Information. Victrelis (boceprevir). Schering-Plough Corporation, Kenilworth, NJ. | ||||
| 34 | Product Information. Stribild (cobicistat/elvitegravir/emtricitabine/tenofov). Gilead Sciences, Foster City, CA. | ||||
| 35 | Product Information. Prezista (darunavir). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 36 | Agencia Espaola de Medicamentos y Productos Sanitarios Healthcare "Centro de informacion online de medicamentos de la AEMPS - CIMA.". | ||||
| 37 | Product Information. Vaprisol (conivaptan). Cumberland Pharmaceuticals Inc, Nashville, TN. | ||||
| 38 | Product Information. Alunbrig (brigatinib). Ariad Pharmaceuticals Inc, Cambridge, MA. | ||||
| 39 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 40 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 41 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 42 | Product Information. Exjade (deferasirox). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 43 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 44 | Product Information. Thalomid (thalidomide). Celgene Corporation, Warren, NJ. | ||||
| 45 | Product Information. Tasigna (nilotinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 46 | Product Information. Sprycel (dasatinib). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 47 | Abernethy DR, Greenblatt DJ, Morse DS, Shader RI "Interaction of propoxyphene with diazepam, alprazolam and lorazepam." Br J Clin Pharmacol 19 (1985): 51-7. [PMID: 2858217] | ||||
| 48 | Sekar M, Mimpriss TJ "Buprenorphine, benzodiazepines and prolonged respiratory depression." Anaesthesia 42 (1987): 567-8. [PMID: 3592200] | ||||
| 49 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 50 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 51 | Product Information. Prograf (tacrolimus). Fujisawa, Deerfield, IL. | ||||
| 52 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
| 53 | Product Information. Zanaflex (tizanidine). Acorda Therapeutics, Hawthorne, NY. | ||||
