Details of the Drug
General Information of Drug (ID: DMZHUO5)
| Drug Name |
Phenylephrine
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Cyclomydril; Dilatair; Dionephrine; Doktors; Duration; Fenilefrina; Isophrim; Isophrin; Mesaton; Mesatone; Mesatonum; Metaoxedrin; Metaoxedrine; Metaoxedrinum; Metasympatol; Metasynephrine; Metsatonum; Mezaton; Mydfrin; Neofrin; Neosynephrine; Nostril; Ocugestrin; Phenoptic; Phenylephrinum; Spersaphrine; Visadron; Alcon Efrin; Isopto Frin; Minims Phenylephrine; Nostril Spray Pump; Nostril Spray Pump Mild; Phenylephrine Minims; Prefrin Liquifilm; Relief Eye Drops for Red Eyes; Alconefrin Nasal Drops 12; Alconefrin Nasal Drops 25; Alconefrin Nasal Drops 50; Alconefrin Nasal Spray 25; Ah-Chew; Ak-dilate; Ak-nefrin; Fenilefrina [INN-Spanish]; I-Phrine; L-Phenylephedrine; L-Phenylephrine; M-Methylaminoethanolphenol; M-Oxedrine; M-Sympathol; M-Sympatol; M-Synephrine; Mydfrin (TN); Neo-Synephrine; Neo-Synephrine Nasal Drops; Neo-Synephrine Nasal Jelly; Neo-Synephrine Nasal Spray; Ocu-Phrin Sterile Eye Drops; Phenylephrine (INN); Phenylephrine Minims (TN); Phenylephrine [INN:BAN]; Phenylephrinum [INN-Latin]; R(-)-Phenylephrine; L-(3-Hydroxyphenyl)-N-methylethanolamine; L-1-(m-Hydroxyphenyl)-2-methylaminoethanol; L-m-Hydroxy-alpha-((methylamino)methyl)benzyl alcohol; L-alpha-Hydroxy-beta-methylamino-3-hydroxy-L-ethylbenzene; Tannins, compds. with (R)-3-hydroxy-alpha-((methylamino)methyl)benzenemethanol; Benzenemethanol, 3-hydroxy-alpha-((methylamino)methyl)-, (R)-(9CI); (-)-m-Hydroxy-alpha-(methylaminomethyl)benzyl alcohol; (R)-2-Hydroxy-2-(3-hydroxyphenyl)-N-methylethylamine; (R)-3-Hydroxy-alpha-((methylamino)methyl)benzenemethanol; 3-[(1R)-1-hydroxy-2-(methylamino)ethyl]phenol
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Ophthalmologicals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
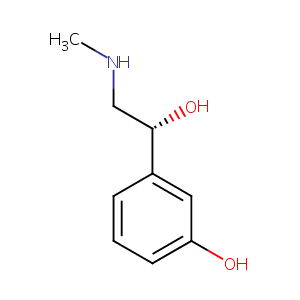 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 167.2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Allergic rhinitis | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | CA08.0 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Phenylephrine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Isoproterenol FDA Label | ||||
|---|---|---|---|---|---|
| 2 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800019494) | ||||
| 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 485). | ||||
| 4 | BDDCS applied to over 900 drugs | ||||
| 5 | FDA Approved Drug Products: Phenylephrine Solution for Intravenous Injection | ||||
| 6 | Pharmacokinetics of oral decongestants. Pharmacotherapy. 1993 Nov-Dec;13(6 Pt 2):116S-128S; discussion 143S-146S. | ||||
| 7 | Forgue ST, Patterson BE, Bedding AW, Payne CD, Phillips DL, Wrishko RE, Mitchell MI: Tadalafil pharmacokinetics in healthy subjects. Br J Clin Pharmacol. 2006 Mar;61(3):280-8. doi: 10.1111/j.1365-2125.2005.02553.x. | ||||
| 8 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 9 | MMP-2 induced vein relaxation via inhibition of [Ca2+]e-dependent mechanisms of venous smooth muscle contraction. Role of RGD peptides. J Surg Res. 2010 Apr;159(2):755-64. | ||||
| 10 | Carvedilol selectively inhibits oscillatory intracellular calcium changes evoked by human alpha1D- and alpha1B-adrenergic receptors. Cardiovasc Res. 2004 Sep 1;63(4):662-72. doi: 10.1016/j.cardiores.2004.05.014. | ||||
| 11 | Influence of angiotensin-I-converting-enzyme insertion/deletion gene polymorphism on perioperative hemodynamics after coronary bypass graft surgery. J Cardiovasc Surg (Torino). 2008 Apr;49(2):255-60. | ||||
| 12 | Carvedilol-induced antagonism of angiotensin II: a matter of alpha1-adrenoceptor blockade. J Hypertens. 2006 Jul;24(7):1355-63. doi: 10.1097/01.hjh.0000234116.17778.63. | ||||
| 13 | Phenylephrine induces necroptosis and apoptosis in corneal epithelial cells dose- and time-dependently. Toxicology. 2019 Dec 1;428:152305. doi: 10.1016/j.tox.2019.152305. Epub 2019 Oct 9. | ||||
| 14 | Ban TA "Drug interactions with psychoactive drugs." Dis Nerv Syst 36 (1975): 164-6. [PMID: 1116424] | ||||
| 15 | Product Information. Northera (droxidopa). Chelsea Therapeutics Inc, Charlotte, NC. | ||||
| 16 | Hendershot PE, Antal EJ, Welshman IR, Batts DH, Hopkins NK "Linezolid: pharmacokinetic and pharmacodynamic evaluation of coadministration with pseudoephedrine HCl, phenylpropanolamine HCl, and dextromethorpan HBr." J Clin Pharmacol 41 (2001): 563-72. [PMID: 11361053] | ||||
| 17 | Cass E, Kadar D, Stein HA "Hazards of phenylephrine topical medication in persons taking propranolol." Can Med Assoc J 120 (1979): 1261-2. [PMID: 221086] | ||||
| 18 | Product Information. Cymbalta (duloxetine). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 19 | Barthel W, Glusa E, Koth W "Interactions of dihydroergotamine with etilefrine in human leg veins in vitro and in situ." Int J Clin Pharmacol Ther Toxicol 25 (1987): 63-9. [PMID: 2881898] | ||||
| 20 | Product Information. Meridia (sibutramine). Knoll Pharmaceutical Company, Whippany, NJ. | ||||
| 21 | Agencia Espaola de Medicamentos y Productos Sanitarios Healthcare "Centro de informacion online de medicamentos de la AEMPS - CIMA.". | ||||
