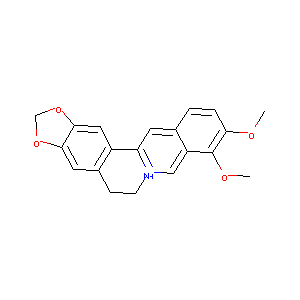
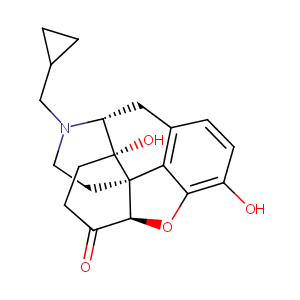
| DOT Name |
DOT ID |
UniProt ID |
Mode of Action |
REF |
|
Cytochrome P450 1A1 (CYP1A1)
|
OTE4EFH8
|
CP1A1_HUMAN
|
Increases Expression
|
[13] |
|
Aryl hydrocarbon receptor (AHR)
|
OTFE4EYE
|
AHR_HUMAN
|
Increases Activity
|
[13] |
|
Insulin-like growth factor 2 mRNA-binding protein 3 (IGF2BP3)
|
OTB97VIK
|
IF2B3_HUMAN
|
Decreases Expression
|
[14] |
|
Protein arginine N-methyltransferase 5 (PRMT5)
|
OTF6IGJS
|
ANM5_HUMAN
|
Increases Expression
|
[8] |
|
Telomerase reverse transcriptase (TERT)
|
OT085VVA
|
TERT_HUMAN
|
Decreases Activity
|
[15] |
|
Serine/threonine-protein kinase Chk1 (CHEK1)
|
OTTTI622
|
CHK1_HUMAN
|
Increases Expression
|
[16] |
|
Histone acetyltransferase type B catalytic subunit (HAT1)
|
OT307KEN
|
HAT1_HUMAN
|
Increases Expression
|
[8] |
|
Lysine-specific demethylase 6B (KDM6B)
|
OTMZVHSG
|
KDM6B_HUMAN
|
Increases Expression
|
[8] |
|
Baculoviral IAP repeat-containing protein 5 (BIRC5)
|
OTILXZYL
|
BIRC5_HUMAN
|
Affects Binding
|
[17] |
|
Lysine-specific demethylase 6A (KDM6A)
|
OTZM3MJJ
|
KDM6A_HUMAN
|
Increases Expression
|
[8] |
|
Bcl-2-like protein 11 (BCL2L11)
|
OTNQQWFJ
|
B2L11_HUMAN
|
Increases Expression
|
[18] |
|
BCL2/adenovirus E1B 19 kDa protein-interacting protein 3-like (BNIP3L)
|
OTJKOMXE
|
BNI3L_HUMAN
|
Increases Expression
|
[19] |
|
Lysine-specific histone demethylase 1A (KDM1A)
|
OT85JXS5
|
KDM1A_HUMAN
|
Increases Expression
|
[8] |
|
Histone H2B type 1-K (H2BC12)
|
OTQ8V0KG
|
H2B1K_HUMAN
|
Decreases Expression
|
[8] |
|
Lysine-specific demethylase 4A (KDM4A)
|
OTDN3EGK
|
KDM4A_HUMAN
|
Increases Expression
|
[8] |
|
Nuclear receptor subfamily 1 group I member 2 (NR1I2)
|
OTC5U0N5
|
NR1I2_HUMAN
|
Increases Activity
|
[20] |
|
Serine/threonine-protein kinase Chk2 (CHEK2)
|
OT8ZPCNS
|
CHK2_HUMAN
|
Increases Expression
|
[16] |
|
Histone-lysine N-methyltransferase NSD2 (NSD2)
|
OTQ6SW4R
|
NSD2_HUMAN
|
Decreases Expression
|
[8] |
|
Epidermal growth factor receptor (EGFR)
|
OTAPLO1S
|
EGFR_HUMAN
|
Decreases Expression
|
[7] |
|
Tumor necrosis factor (TNF)
|
OT4IE164
|
TNFA_HUMAN
|
Increases Expression
|
[21] |
|
Collagen alpha-1(I) chain (COL1A1)
|
OTI31178
|
CO1A1_HUMAN
|
Increases Expression
|
[9] |
|
Collagen alpha-1(II) chain (COL2A1)
|
OT5E59C8
|
CO2A1_HUMAN
|
Decreases Expression
|
[9] |
|
Osteocalcin (BGLAP)
|
OTK1YLWQ
|
OSTCN_HUMAN
|
Increases Expression
|
[9] |
|
Cellular tumor antigen p53 (TP53)
|
OTIE1VH3
|
P53_HUMAN
|
Increases Expression
|
[21] |
|
Histone H2A type 1-B/E (H2AC4)
|
OTMFQ662
|
H2A1B_HUMAN
|
Decreases Expression
|
[8] |
|
Histone H2B type 1-J (H2BC11)
|
OTGM083T
|
H2B1J_HUMAN
|
Decreases Expression
|
[8] |
|
Cathepsin D (CTSD)
|
OTQZ36F3
|
CATD_HUMAN
|
Decreases Cleavage
|
[19] |
|
Procathepsin L (CTSL)
|
OTYTUW29
|
CATL1_HUMAN
|
Decreases Cleavage
|
[19] |
|
Insulin-like growth factor 1 receptor (IGF1R)
|
OTXJIF13
|
IGF1R_HUMAN
|
Increases Expression
|
[22] |
|
72 kDa type IV collagenase (MMP2)
|
OT5NIWA2
|
MMP2_HUMAN
|
Decreases Activity
|
[23] |
|
SPARC (SPARC)
|
OTPN90H0
|
SPRC_HUMAN
|
Increases Expression
|
[9] |
|
Heme oxygenase 1 (HMOX1)
|
OTC1W6UX
|
HMOX1_HUMAN
|
Increases Expression
|
[22] |
|
Poly polymerase 1 (PARP1)
|
OT310QSG
|
PARP1_HUMAN
|
Increases Cleavage
|
[24] |
|
Histone H2A type 1 (H2AC11)
|
OTN9P0BO
|
H2A1_HUMAN
|
Decreases Expression
|
[8] |
|
Histone H2B type F-M (H2BW2)
|
OT4X4HHV
|
H2BFM_HUMAN
|
Decreases Expression
|
[8] |
|
Apoptosis regulator Bcl-2 (BCL2)
|
OT9DVHC0
|
BCL2_HUMAN
|
Decreases Expression
|
[21] |
|
Osteopontin (SPP1)
|
OTJGC23Y
|
OSTP_HUMAN
|
Increases Expression
|
[9] |
|
DNA topoisomerase 1 (TOP1)
|
OT51O0CF
|
TOP1_HUMAN
|
Decreases Activity
|
[16] |
|
Cyclin-dependent kinase 4 (CDK4)
|
OT7EP05T
|
CDK4_HUMAN
|
Decreases Expression
|
[24] |
|
Hepatocyte growth factor (HGF)
|
OTGHUA23
|
HGF_HUMAN
|
Increases Expression
|
[25] |
|
Sucrase-isomaltase, intestinal (SI)
|
OTUP724T
|
SUIS_HUMAN
|
Decreases Expression
|
[26] |
|
Matrix metalloproteinase-9 (MMP9)
|
OTB2QDAV
|
MMP9_HUMAN
|
Decreases Activity
|
[7] |
|
Fatty acid-binding protein, adipocyte (FABP4)
|
OT3DKFOU
|
FABP4_HUMAN
|
Decreases Expression
|
[9] |
|
Histone H2AX (H2AX)
|
OT18UX57
|
H2AX_HUMAN
|
Decreases Expression
|
[8] |
|
Aggrecan core protein (ACAN)
|
OTUOCW8K
|
PGCA_HUMAN
|
Decreases Expression
|
[9] |
|
Platelet glycoprotein 4 (CD36)
|
OT5CZWKY
|
CD36_HUMAN
|
Increases Expression
|
[27] |
|
CCAAT/enhancer-binding protein beta (CEBPB)
|
OTM9MQIA
|
CEBPB_HUMAN
|
Increases Expression
|
[27] |
|
Cadherin-2 (CDH2)
|
OTH0Y56P
|
CADH2_HUMAN
|
Decreases Expression
|
[9] |
|
Acetylcholinesterase (ACHE)
|
OT2H8HG6
|
ACES_HUMAN
|
Decreases Activity
|
[28] |
|
Histone H2B type 1-O (H2BC17)
|
OT8ED05P
|
H2B1O_HUMAN
|
Decreases Expression
|
[8] |
|
G1/S-specific cyclin-D1 (CCND1)
|
OT8HPTKJ
|
CCND1_HUMAN
|
Decreases Expression
|
[24] |
|
G1/S-specific cyclin-E1 (CCNE1)
|
OTLD7UID
|
CCNE1_HUMAN
|
Decreases Expression
|
[24] |
|
Cyclin-dependent kinase 2 (CDK2)
|
OTB5DYYZ
|
CDK2_HUMAN
|
Decreases Expression
|
[24] |
|
Tumor necrosis factor receptor superfamily member 6 (FAS)
|
OTP9XG86
|
TNR6_HUMAN
|
Increases Expression
|
[21] |
|
DNA cytosine-5)-methyltransferase 1 (DNMT1)
|
OTM2DGTK
|
DNMT1_HUMAN
|
Decreases Expression
|
[8] |
|
G1/S-specific cyclin-D2 (CCND2)
|
OTDULQF9
|
CCND2_HUMAN
|
Decreases Expression
|
[24] |
|
RAC-alpha serine/threonine-protein kinase (AKT1)
|
OT8H2YY7
|
AKT1_HUMAN
|
Decreases Phosphorylation
|
[7] |
|
Catenin beta-1 (CTNNB1)
|
OTZ932A3
|
CTNB1_HUMAN
|
Increases Expression
|
[9] |
|
Sterol regulatory element-binding protein 1 (SREBF1)
|
OTWBRPAI
|
SRBP1_HUMAN
|
Decreases Expression
|
[9] |
|
Peroxisome proliferator-activated receptor gamma (PPARG)
|
OTHMARHO
|
PPARG_HUMAN
|
Decreases Expression
|
[9] |
|
Cyclin-dependent kinase inhibitor 1 (CDKN1A)
|
OTQWHCZE
|
CDN1A_HUMAN
|
Increases Expression
|
[24] |
|
Caspase-3 (CASP3)
|
OTIJRBE7
|
CASP3_HUMAN
|
Increases Expression
|
[21] |
|
Growth/differentiation factor 5 (GDF5)
|
OTOV8S81
|
GDF5_HUMAN
|
Decreases Expression
|
[9] |
|
Nicotinamide phosphoribosyltransferase (NAMPT)
|
OTVJR3GL
|
NAMPT_HUMAN
|
Decreases Expression
|
[18] |
|
Collagenase 3 (MMP13)
|
OTY8BZIE
|
MMP13_HUMAN
|
Increases Expression
|
[9] |
|
Cyclin-dependent kinase inhibitor 1B (CDKN1B)
|
OTNY5LLZ
|
CDN1B_HUMAN
|
Increases Expression
|
[24] |
|
Tumor necrosis factor ligand superfamily member 6 (FASLG)
|
OTZARCHH
|
TNFL6_HUMAN
|
Increases Expression
|
[21] |
|
Transcription factor SOX-9 (SOX9)
|
OTVDJFGN
|
SOX9_HUMAN
|
Decreases Expression
|
[9] |
|
CCAAT/enhancer-binding protein alpha (CEBPA)
|
OTOM9OE4
|
CEBPA_HUMAN
|
Decreases Expression
|
[9] |
|
Glycogen synthase kinase-3 beta (GSK3B)
|
OTL3L14B
|
GSK3B_HUMAN
|
Increases Phosphorylation
|
[22] |
|
Cyclin-dependent kinase inhibitor 1C (CDKN1C)
|
OTASTJ3Q
|
CDN1C_HUMAN
|
Increases Expression
|
[9] |
|
Isocitrate dehydrogenase subunit alpha, mitochondrial (IDH3A)
|
OT5QQB5L
|
IDH3A_HUMAN
|
Decreases Expression
|
[29] |
|
Caspase-9 (CASP9)
|
OTD4RFFG
|
CASP9_HUMAN
|
Increases Cleavage
|
[24] |
|
Protein arginine N-methyltransferase 2 (PRMT2)
|
OT63JZCI
|
ANM2_HUMAN
|
Increases Expression
|
[8] |
|
Integrin alpha-1 (ITGA1)
|
OTDITIST
|
ITA1_HUMAN
|
Increases Expression
|
[9] |
|
Histone H2B type 1-D (H2BC5)
|
OTXDTDHN
|
H2B1D_HUMAN
|
Decreases Expression
|
[8] |
|
Small ribosomal subunit protein eS6 (RPS6)
|
OTT4D1LN
|
RS6_HUMAN
|
Decreases Phosphorylation
|
[30] |
|
Histone H4 (H4C1)
|
OTB71W46
|
H4_HUMAN
|
Decreases Expression
|
[8] |
|
Histone H2B type 1-C/E/F/G/I (H2BC10)
|
OTLQH0D6
|
H2B1C_HUMAN
|
Decreases Expression
|
[8] |
|
Histone H3.1 (H3C1)
|
OTGBGOZW
|
H31_HUMAN
|
Decreases Expression
|
[8] |
|
Histone H3.3 (H3-3B)
|
OT9XHQ3C
|
H33_HUMAN
|
Decreases Expression
|
[8] |
|
Cytochrome c (CYCS)
|
OTBFALJD
|
CYC_HUMAN
|
Affects Localization
|
[24] |
|
Cyclin-dependent kinase 6 (CDK6)
|
OTR95N0X
|
CDK6_HUMAN
|
Decreases Expression
|
[24] |
|
Collagen alpha-1(X) chain (COL10A1)
|
OTC4G2YC
|
COAA1_HUMAN
|
Increases Expression
|
[9] |
|
Apoptosis regulator BAX (BAX)
|
OTAW0V4V
|
BAX_HUMAN
|
Increases Expression
|
[21] |
|
Bcl-2-like protein 1 (BCL2L1)
|
OTRC5K9O
|
B2CL1_HUMAN
|
Decreases Expression
|
[24] |
|
Histone acetyltransferase p300 (EP300)
|
OTL8QJDX
|
EP300_HUMAN
|
Increases Expression
|
[8] |
|
BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 (BNIP3)
|
OT4SO7J4
|
BNIP3_HUMAN
|
Increases Expression
|
[19] |
|
TNF receptor-associated factor 1 (TRAF1)
|
OTTLM5RU
|
TRAF1_HUMAN
|
Increases Expression
|
[21] |
|
Sequestosome-1 (SQSTM1)
|
OTGY5D5J
|
SQSTM_HUMAN
|
Increases Expression
|
[19] |
|
Runt-related transcription factor 2 (RUNX2)
|
OT97RQQM
|
RUNX2_HUMAN
|
Increases Expression
|
[9] |
|
Caspase-8 (CASP8)
|
OTA8TVI8
|
CASP8_HUMAN
|
Increases Activity
|
[21] |
|
Nuclear receptor subfamily 1 group I member 3 (NR1I3)
|
OTS3SGH7
|
NR1I3_HUMAN
|
Increases Activity
|
[29] |
|
Histone-lysine N-methyltransferase SETDB1 (SETDB1)
|
OTWVUA1B
|
SETB1_HUMAN
|
Increases Expression
|
[8] |
|
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
|
OT0HENJ5
|
NF2L2_HUMAN
|
Increases Activity
|
[29] |
|
Histone H3.1t (H3-4)
|
OTY6ITYF
|
H31T_HUMAN
|
Decreases Expression
|
[8] |
|
Histone H2A type 2-A (H2AC18)
|
OTCE5JC7
|
H2A2A_HUMAN
|
Decreases Expression
|
[8] |
|
Histone H2A type 3 (H2AC25)
|
OTPO62JI
|
H2A3_HUMAN
|
Decreases Expression
|
[8] |
|
Actin-histidine N-methyltransferase (SETD3)
|
OTO5RAU2
|
SETD3_HUMAN
|
Increases Expression
|
[8] |
|
E3 ubiquitin-protein ligase DZIP3 (DZIP3)
|
OTOFK9KG
|
DZIP3_HUMAN
|
Increases Expression
|
[8] |
|
FUN14 domain-containing protein 1 (FUNDC1)
|
OTA6IVKQ
|
FUND1_HUMAN
|
Increases Expression
|
[19] |
|
Thiosulfate:glutathione sulfurtransferase (TSTD1)
|
OT5DMKFX
|
TSTD1_HUMAN
|
Increases Expression
|
[8] |
|
N-lysine methyltransferase SETD6 (SETD6)
|
OTH5APN1
|
SETD6_HUMAN
|
Increases Expression
|
[8] |
|
Transcription factor Sp7 (SP7)
|
OT07ETZT
|
SP7_HUMAN
|
Increases Expression
|
[9] |
|
Histone-lysine N-methyltransferase SETD7 (SETD7)
|
OTT49OXF
|
SETD7_HUMAN
|
Increases Expression
|
[8] |
|
Histone deacetylase 2 (HDAC2)
|
OTUYVFKN
|
HDAC2_HUMAN
|
Decreases Expression
|
[8] |
|
CREB-binding protein (CREBBP)
|
OTPA4QGM
|
CBP_HUMAN
|
Increases Expression
|
[8] |
|
Proteoglycan 4 (PRG4)
|
OT34NR3N
|
PRG4_HUMAN
|
Decreases Expression
|
[9] |
|
Histone H2A type 1-C (H2AC6)
|
OTF3YK7V
|
H2A1C_HUMAN
|
Decreases Expression
|
[8] |
|
Histone H2B type 1-H (H2BC9)
|
OTQ9XX72
|
H2B1H_HUMAN
|
Decreases Expression
|
[8] |
|
NAD-dependent protein deacetylase sirtuin-1 (SIRT1)
|
OTAYZMOY
|
SIR1_HUMAN
|
Decreases Expression
|
[18] |
|
Aurora kinase B (AURKB)
|
OTIY4VHU
|
AURKB_HUMAN
|
Decreases Expression
|
[8] |
|
Histone H2A type 1-H (H2AC12)
|
OTLXVB97
|
H2A1H_HUMAN
|
Decreases Expression
|
[8] |
|
Protein arginine N-methyltransferase 6 (PRMT6)
|
OT5V3XIN
|
ANM6_HUMAN
|
Increases Expression
|
[8] |
|
Bcl-2-binding component 3, isoforms 3/4 (BBC3)
|
OTUAXDAY
|
BBC3B_HUMAN
|
Increases Expression
|
[18] |
|
Histone-lysine N-methyltransferase SETDB2 (SETDB2)
|
OTBVVP9Q
|
SETB2_HUMAN
|
Increases Expression
|
[8] |
|
Protein arginine N-methyltransferase 1 (PRMT1)
|
OTN8GTXW
|
ANM1_HUMAN
|
Decreases Expression
|
[8] |
|
Histone H2B type 1-N (H2BC15)
|
OTU39CFZ
|
H2B1N_HUMAN
|
Decreases Expression
|
[8] |
|
Histone H2A type 1-J (H2AC14)
|
OT5Y4MMC
|
H2A1J_HUMAN
|
Decreases Expression
|
[8] |
|
Histone H2B type 1-M (H2BC14)
|
OTSDZZZY
|
H2B1M_HUMAN
|
Decreases Expression
|
[8] |
|
Histone H2B type 1-L (H2BC13)
|
OTIS8L6R
|
H2B1L_HUMAN
|
Decreases Expression
|
[8] |
|
Osteomodulin (OMD)
|
OTFRELBB
|
OMD_HUMAN
|
Increases Expression
|
[9] |
|
Histone deacetylase 8 (HDAC8)
|
OT5L4RYX
|
HDAC8_HUMAN
|
Decreases Expression
|
[8] |
|
Histone-lysine N-methyltransferase SETD2 (SETD2)
|
OTQW463T
|
SETD2_HUMAN
|
Increases Expression
|
[8] |
|
Histone-lysine N-methyltransferase SETD5 (SETD5)
|
OTRPAVEO
|
SETD5_HUMAN
|
Increases Expression
|
[8] |
|
Histone-lysine N-methyltransferase SMYD3 (SMYD3)
|
OT9YR20G
|
SMYD3_HUMAN
|
Affects Expression
|
[8] |
|
N-lysine methyltransferase KMT5A (KMT5A)
|
OTW2A5YY
|
KMT5A_HUMAN
|
Increases Expression
|
[8] |
|
Protein arginine N-methyltransferase 8 (PRMT8)
|
OT3MRK62
|
ANM8_HUMAN
|
Increases Expression
|
[8] |
|
NAD-dependent protein deacetylase sirtuin-3, mitochondrial (SIRT3)
|
OTMEF544
|
SIR3_HUMAN
|
Decreases Expression
|
[18] |
|
Protein arginine N-methyltransferase 7 (PRMT7)
|
OTE73PO1
|
ANM7_HUMAN
|
Increases Expression
|
[8] |
|
DNA (DNMT3B)
|
OTZ0JCNP
|
DNM3B_HUMAN
|
Decreases Expression
|
[8] |
|
Lysine-specific demethylase 5B (KDM5B)
|
OT5DL94T
|
KDM5B_HUMAN
|
Increases Expression
|
[8] |
|
Histone deacetylase 9 (HDAC9)
|
OTO8O0LF
|
HDAC9_HUMAN
|
Decreases Expression
|
[8] |
|
Aurora kinase C (AURKC)
|
OTJMOW9V
|
AURKC_HUMAN
|
Increases Expression
|
[8] |
|
Histone deacetylase 5 (HDAC5)
|
OTWG387P
|
HDAC5_HUMAN
|
Increases Expression
|
[8] |
|
Long-chain fatty acid transport protein 5 (SLC27A5)
|
OT4OK511
|
S27A5_HUMAN
|
Decreases Expression
|
[27] |
|
DNA topoisomerase 2-alpha (TOP2A)
|
OT6LPS08
|
TOP2A_HUMAN
|
Affects Response To Substance
|
[16] |
| ------------------------------------------------------------------------------------ |
|
|
|
|