| 1 |
ClinicalTrials.gov (NCT00000512) Familial Atherosclerosis Treatment Study
|
| 2 |
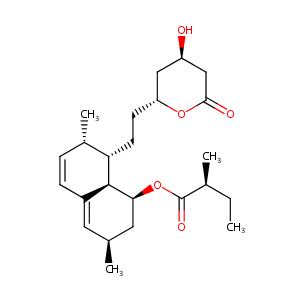
Lovastatin FDA Label
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2739).
|
| 4 |
Clinical pipeline report, company report or official report of CardioPharma Wilmington.
|
| 5 |
Colestipol FDA Label
|
| 6 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 7 |
Microarray and biochemical analysis of lovastatin-induced apoptosis of squamous cell carcinomas. Neoplasia. 2002 Jul-Aug;4(4):337-46.
|
| 8 |
Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64.
|
| 9 |
A novel human hepatic organic anion transporting polypeptide (OATP2). Identification of a liver-specific human organic anion transporting polypeptide and identification of rat and human hydroxymethylglutaryl-CoA reductase inhibitor transporters. J Biol Chem. 1999 Dec 24;274(52):37161-8.
|
| 10 |
Metabolic interactions with statins. Tidsskr Nor Laegeforen. 2001 Jan 20;121(2):189-93.
|
| 11 |
Pharmacogenomics of statins: understanding susceptibility to adverse effects. Pharmgenomics Pers Med. 2016 Oct 3;9:97-106.
|
| 12 |
Paraoxonases-1, -2 and -3: what are their functions? Chem Biol Interact. 2016 Nov 25;259(Pt B):51-62.
|
| 13 |
Examination of 209 drugs for inhibition of cytochrome P450 2C8. J Clin Pharmacol. 2005 Jan;45(1):68-78.
|
| 14 |
Receptor-dependent regulation of the CYP3A4 gene. Toxicology. 2002 Dec 27;181-182:199-202.
|
| 15 |
Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res. 2005 Jun;46(6):1239-47. doi: 10.1194/jlr.M400511-JLR200. Epub 2005 Mar 16.
|
| 16 |
Differential interaction of 3-hydroxy-3-methylglutaryl-coa reductase inhibitors with ABCB1, ABCC2, and OATP1B1. Drug Metab Dispos. 2005 Apr;33(4):537-46.
|
| 17 |
Desmosterol can replace cholesterol in sustaining cell proliferation and regulating the SREBP pathway in a sterol-Delta24-reductase-deficient cell line. Biochem J. 2009 May 13;420(2):305-15.
|
| 18 |
Lovastatin lactone elicits human lung cancer cell apoptosis via a COX-2/PPAR-dependent pathway. Oncotarget. 2016 Mar 1;7(9):10345-62. doi: 10.18632/oncotarget.7213.
|
| 19 |
Lovastatin induces apoptosis of anaplastic thyroid cancer cells via inhibition of protein geranylgeranylation and de novo protein synthesis. Endocrinology. 2003 Sep;144(9):3852-9. doi: 10.1210/en.2003-0098.
|
| 20 |
Statins activate the mitochondrial pathway of apoptosis in human lymphoblasts and myeloma cells. Carcinogenesis. 2005 May;26(5):883-91. doi: 10.1093/carcin/bgi036. Epub 2005 Feb 10.
|
| 21 |
Pravastatin inhibited the cholesterol synthesis in human hepatoma cell line Hep G2 less than simvastatin and lovastatin, which is reflected in the upregulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase and squalene synthase. Biochem Pharmacol. 1993 Jun 9;45(11):2203-8.
|
| 22 |
A multifactorial approach to hepatobiliary transporter assessment enables improved therapeutic compound development. Toxicol Sci. 2013 Nov;136(1):216-41.
|
| 23 |
Survivin down-regulation plays a crucial role in 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor-induced apoptosis in cancer. J Biol Chem. 2007 Jul 6;282(27):19273-81. doi: 10.1074/jbc.M610350200. Epub 2007 May 1.
|
| 24 |
Statins induce apoptosis in ovarian cancer cells through activation of JNK and enhancement of Bim expression. Cancer Chemother Pharmacol. 2009 May;63(6):997-1005. doi: 10.1007/s00280-008-0830-7. Epub 2008 Sep 3.
|
| 25 |
Combining prenylation inhibitors causes synergistic cytotoxicity, apoptosis and disruption of RAS-to-MAP kinase signalling in multiple myeloma cells. Br J Haematol. 2005 Sep;130(6):912-25. doi: 10.1111/j.1365-2141.2005.05696.x.
|
| 26 |
Effect of atorvastatin, simvastatin, and lovastatin on the metabolism of cholesterol and triacylglycerides in HepG2 cells. Biochem Pharmacol. 2001 Dec 1;62(11):1545-55. doi: 10.1016/s0006-2952(01)00790-0.
|
| 27 |
Lovastatin, a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, induces apoptosis and differentiation in human anaplastic thyroid carcinoma cells. J Clin Endocrinol Metab. 2003 Jul;88(7):3021-6. doi: 10.1210/jc.2002-021834.
|
| 28 |
Simvastatin and lovastatin inhibit breast cell invasion induced by H-Ras. Oncol Rep. 2009 May;21(5):1317-22. doi: 10.3892/or_00000357.
|
| 29 |
Simvastatin prevents skeletal metastasis of breast cancer by an antagonistic interplay between p53 and CD44. J Biol Chem. 2011 Apr 1;286(13):11314-27. doi: 10.1074/jbc.M110.193714. Epub 2011 Jan 3.
|
| 30 |
Protection against the Neurotoxic Effects of -Amyloid Peptide on Cultured Neuronal Cells by Lovastatin Involves Elevated Expression of 7 Nicotinic Acetylcholine Receptors and Activating Phosphorylation of Protein Kinases. Am J Pathol. 2018 Apr;188(4):1081-1093. doi: 10.1016/j.ajpath.2017.11.020. Epub 2018 Jan 16.
|
| 31 |
Lovastatin inhibits G1/S transition of normal human B-lymphocytes independent of apoptosis. Exp Cell Res. 1999 Oct 10;252(1):144-53. doi: 10.1006/excr.1999.4608.
|
| 32 |
Lovastatin induces neuronal differentiation and apoptosis of embryonal carcinoma and neuroblastoma cells: enhanced differentiation and apoptosis in combination with dbcAMP. Mol Cell Biochem. 2010 Dec;345(1-2):1-11. doi: 10.1007/s11010-010-0553-z. Epub 2010 Aug 9.
|
| 33 |
Simvastatin-dependent up-regulation of heme oxygenase-1 via mRNA stabilization in human endothelial cells. Eur J Pharm Sci. 2010 Sep 11;41(1):118-24. doi: 10.1016/j.ejps.2010.05.021. Epub 2010 Jun 8.
|
| 34 |
Lovastatin augments apoptosis induced by chemotherapeutic agents in colon cancer cells. Clin Cancer Res. 1999 Aug;5(8):2223-9.
|
| 35 |
Apoptosis and cell-cycle arrest in human and murine tumor cells are initiated by isoprenoids. J Nutr. 1999 Apr;129(4):804-13. doi: 10.1093/jn/129.4.804.
|
| 36 |
In vitro mechanisms of lovastatin on lung cancer cell lines as a potential chemopreventive agent. Lung. 2008 Jan-Feb;186(1):45-54. doi: 10.1007/s00408-007-9053-7. Epub 2007 Nov 22.
|
| 37 |
Increased PTEN expression due to transcriptional activation of PPARgamma by Lovastatin and Rosiglitazone. Int J Cancer. 2006 May 15;118(10):2390-8. doi: 10.1002/ijc.21799.
|
| 38 |
Indirect co-cultivation of HepG2 with differentiated THP-1 cells induces AHR signalling and release of pro-inflammatory cytokines. Toxicol In Vitro. 2020 Oct;68:104957. doi: 10.1016/j.tiv.2020.104957. Epub 2020 Jul 30.
|
| 39 |
Synergistic interaction of lovastatin and paclitaxel in human cancer cells. Mol Cancer Ther. 2001 Dec;1(2):141-9.
|
| 40 |
Lovastatin protects human neurons against Abeta-induced toxicity and causes activation of beta-catenin-TCF/LEF signaling. Neurosci Lett. 2007 Feb 2;412(3):211-6. doi: 10.1016/j.neulet.2006.07.045. Epub 2007 Jan 17.
|
| 41 |
Lovastatin sensitized human glioblastoma cells to TRAIL-induced apoptosis. J Neurooncol. 2008 Feb;86(3):273-83. doi: 10.1007/s11060-007-9475-3. Epub 2007 Oct 11.
|
| 42 |
Lovastatin-induced apoptosis in thyroid cells: involvement of cytochrome c and lamin B. Eur J Endocrinol. 2001 Nov;145(5):645-50. doi: 10.1530/eje.0.1450645.
|
| 43 |
Inhibitory effects of clinical reagents having anti-oxidative activity on transforming growth factor-1-induced expression of -smooth muscle actin in human fetal lung fibroblasts. J Toxicol Sci. 2011;36(6):733-40. doi: 10.2131/jts.36.733.
|
| 44 |
Systems pharmacological analysis of drugs inducing stevens-johnson syndrome and toxic epidermal necrolysis. Chem Res Toxicol. 2015 May 18;28(5):927-34. doi: 10.1021/tx5005248. Epub 2015 Apr 3.
|
| 45 |
Comparative evaluation of HERG currents and QT intervals following challenge with suspected torsadogenic and nontorsadogenic drugs. J Pharmacol Exp Ther. 2006 Mar;316(3):1098-106. doi: 10.1124/jpet.105.093393. Epub 2005 Nov 8.
|
| 46 |
Association of CYP1A1 and CYP1B1 inhibition in in vitro assays with drug-induced liver injury. J Toxicol Sci. 2021;46(4):167-176. doi: 10.2131/jts.46.167.
|
| 47 |
NCI60 cancer cell line panel data and RNAi analysis help identify EAF2 as a modulator of simvastatin and lovastatin response in HCT-116 cells. PLoS One. 2011 Apr 4;6(4):e18306. doi: 10.1371/journal.pone.0018306.
|
| 48 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
|
|
|
|
|
|