Details of the Drug
General Information of Drug (ID: DMBS632)
| Drug Name |
Buspirone
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Ansial; Ansiced; Anxiron; Axoren; Bespar; Buspirona; Buspironum; Buspisal; Ansial (TN); Ansiced (TN); Anxiron (TN); Axoren (TN); Bespar (TN); BuSpar (TN); Buspimen (TN); Buspinol (TN); Buspiron (TN); Buspirona [INN-Spanish]; Buspirone (INN); Buspirone [INN:BAN]; Buspirone-MDTS; Buspironum [INN-Latin]; Buspisal (TN); Gen-Buspirone; Narol (TN); Sorbon (TN); Spamilan (TN); Spitomin (TN); Gen-Buspirone (TN); MJ-9022-1; N-(4-(4-(2-pyrimidinyl)-1-piperazinyl)butyl)-1-cyclopentanediacetamide; 8-(4-(4-(2-Pyrimidinyl)-1-piperizinyl)butyl)-8-azaspiro(4,5)decane-7,9-dione; 8-[4-(4-Pyrimidin-2-yl-piperazin-1-yl)-butyl]-8-aza-spiro[4.5]decane-7,9-dione; 8-[4-(4-pyrimidin-2-ylpiperazin-1-yl)butyl]-8-azaspiro[4.5]decane-7,9-dione; 8-{4-[4-(pyrimidin-2-yl)piperazin-1-yl]butyl}-8-azaspiro[4.5]decane-7,9-dione
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antianxiety Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
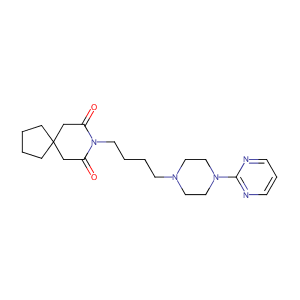 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 385.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Anxiety disorder | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 6B00-6B0Z | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Buspirone
Coadministration of a Drug Treating the Disease Different from Buspirone (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 36). | ||||
|---|---|---|---|---|---|
| 2 | Seltzer B: Donepezil: a review. Expert Opin Drug Metab Toxicol. 2005 Oct;1(3):527-36. doi: 10.1517/17425255.1.3.527 . | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Mahmood I, Sahajwalla C: Clinical pharmacokinetics and pharmacodynamics of buspirone, an anxiolytic drug. Clin Pharmacokinet. 1999 Apr;36(4):277-87. doi: 10.2165/00003088-199936040-00003. | ||||
| 5 | BuSpar? (buspirone HCl) FDA Label | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | Interactions between corticotropin-releasing hormone and serotonin: implications for the aetiology and treatment of anxiety disorders. Handb Exp Pharmacol. 2005;(169):181-204. | ||||
| 8 | Effects of cytochrome P450 (CYP) 3A4 inhibitors on the anxiolytic action of tandospirone in rat contextual conditioned fear. Prog Neuropsychopharmacol Biol Psychiatry. 2007 May 9;31(4):926-31. | ||||
| 9 | Cytochrome P450 3A-mediated metabolism of buspirone in human liver microsomes. Drug Metab Dispos. 2005 Apr;33(4):500-7. | ||||
| 10 | Drug Interactions Flockhart Table | ||||
| 11 | The bacterial P450 BM3: a prototype for a biocatalyst with human P450 activities. Trends Biotechnol. 2007 Jul;25(7):289-98. | ||||
| 12 | acillus megaterium SF185 spores exert protective effects against oxidative stress in vivo and in vitro. Sci Rep. 2019 Aug 19;9(1):12082. | ||||
| 13 | Hormonal responses to the 5-HT1A agonist buspirone in remitted endogenous depressive patients after long-term imipramine treatment. Psychoneuroendocrinology. 2010 May;35(4):481-9. doi: 10.1016/j.psyneuen.2009.08.012. Epub 2009 Sep 16. | ||||
| 14 | Effect of common medications on the expression of SARS-CoV-2 entry receptors in liver tissue. Arch Toxicol. 2020 Dec;94(12):4037-4041. doi: 10.1007/s00204-020-02869-1. Epub 2020 Aug 17. | ||||
| 15 | Kivisto KT, Lamberg TS, Neuvonen PJ "Interactions of buspirone with itraconazole and rifampicin: Effects on the pharmacokinetics of the active 1-(2-pyrimidinyl)-piperazine metabolite of buspirone." Pharmacol Toxicol 84 (1999): 94-7. [PMID: 10068153] | ||||
| 16 | Boyer EW, Shannon M "The serotonin syndrome." N Engl J Med 352 (2005): 1112-20. [PMID: 15784664] | ||||
| 17 | US Food and Drug Administration "FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines requires its strongest warning.". | ||||
| 18 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 19 | Kivisto KT, Lamberg TS, Kantola T, Neuvonen PJ "Plasma buspirone concentrations are greatly increased by erythromycin and itraconazole." Clin Pharmacol Ther 62 (1997): 348-54. [PMID: 9333111] | ||||
| 20 | Achamallah NS "Visual hallucinations after combining fluoxetine and dextromethorphan ." Am J Psychiatry 149 (1992): 1406. [PMID: 1530079] | ||||
| 21 | Bergeron L, Boule M, Perreault S "Serotonin toxicity associated with concomitant use of linezolid." Ann Pharmacother 39 (2005): 956-61. [PMID: 15827071] | ||||
| 22 | Product Information. Tykerb (lapatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 23 | Product Information. Tukysa (tucatinib). Seattle Genetics Inc, Bothell, WA. | ||||
| 24 | US Food and Drug Administration "FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines requires its strongest warning.". | ||||
| 25 | Warrington SJ, Ankier SI, Turner P "Evaluation of possible interactions between ethanol and trazodone or amitriptyline." Neuropsychobiology 15 (1986): 31-7. [PMID: 3725002] | ||||
| 26 | Gill SS, Wright EM, Reilly CS "Pharmacokinetic interaction of propofol and fentanyl: single bolus injection study." Br J Anaesth 65 (1990): 760-5. [PMID: 2265045] | ||||
| 27 | Gilman AG, Rall TW, Nies AS, Taylor P, eds. "Goodman and Gilman's the Pharmacological Basis of Therapeutics. 8th ed." New York, NY: Pergamon Press Inc. (1990):. | ||||
| 28 | Product Information. Kalydeco (ivacaftor). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 29 | Product Information. Prevymis (letermovir). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 30 | Product Information. Emend (aprepitant). Merck & Company Inc, West Point, PA. | ||||
| 31 | Alvine G, Black DW, Tsuang D "Case of delirium secondary to phenelzine/L-tryptophan combination." J Clin Psychiatry 51 (1990): 311. [PMID: 2365671] | ||||
| 32 | Ciraulo DA, Shader RI "Fluoxetine drug-drug interactions: I. Antidepressants and antipsychotics." J Clin Psychopharmacol 10 (1990): 48-50. [PMID: 1968472] | ||||
| 33 | EMEA. European Medicines Agency "EPARs. European Union Public Assessment Reports.". | ||||
| 34 | Product Information. Tazverik (tazemetostat). Epizyme, Inc, Cambridge, MA. | ||||
| 35 | Product Information. Victrelis (boceprevir). Schering-Plough Corporation, Kenilworth, NJ. | ||||
| 36 | Product Information. Incivek (telaprevir). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 37 | Barry M, Mulcahy F, Merry C, Gibbons S, Back D "Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat patients with HIV infection." Clin Pharmacokinet 36 (1999): 289-304. [PMID: 10320951] | ||||
| 38 | Product Information. Agenerase (amprenavir). Glaxo Wellcome, Research Triangle Pk, NC. | ||||
| 39 | Product Information. Stribild (cobicistat/elvitegravir/emtricitabine/tenofov). Gilead Sciences, Foster City, CA. | ||||
| 40 | Product Information. Fortovase (saquinavir) Roche Laboratories, Nutley, NJ. | ||||
| 41 | Product Information. Intelence (etravirine). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 42 | Product Information. Prezista (darunavir). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 43 | Clay PG, Adams MM "Pseudo-Parkinson disease secondary to ritonavir-buspirone interaction." Ann Pharmacother 37 (2003): 202-5. [PMID: 12549947] | ||||
| 44 | Product Information. Vaprisol (conivaptan). Cumberland Pharmaceuticals Inc, Nashville, TN. | ||||
| 45 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 46 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 47 | Product Information. Braftovi (encorafenib). Array BioPharma Inc., Boulder, CO. | ||||
| 48 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 49 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 50 | Product Information. Thalomid (thalidomide). Celgene Corporation, Warren, NJ. | ||||
| 51 | Product Information. Tasigna (nilotinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 52 | Product Information. Sprycel (dasatinib). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 53 | Canadian Pharmacists Association. | ||||
| 54 | Product Information. Rozlytrek (entrectinib). Genentech, South San Francisco, CA. | ||||
| 55 | Chan BSH, Graudins A, Whyte IM, Dawson AH, Braitberg G, Duggin GG "Serotonin syndrome resulting from drug interactions." Med J Aust 169 (1998): 523-5. [PMID: 9861909] | ||||
| 56 | Product Information. Belviq (lorcaserin). Eisai Inc, Teaneck, NJ. | ||||
| 57 | Product Information. Alphagan (brimonidine ophthalmic). Allergan Inc, Irvine, CA. | ||||
| 58 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 59 | Hansen BS, Dam M, Brandt J, et al "Influence of dextropropoxyphene on steady state serum levels and protein binding of three anti-epileptic drugs in man." Acta Neurol Scand 61 (1980): 357-67. [PMID: 6998251] | ||||
| 60 | Sekar M, Mimpriss TJ "Buprenorphine, benzodiazepines and prolonged respiratory depression." Anaesthesia 42 (1987): 567-8. [PMID: 3592200] | ||||
| 61 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 62 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 63 | Product Information. Zokinvy (lonafarnib). Eiger BioPharmaceuticals, Palo Alto, CA. | ||||
| 64 | Product Information. Prograf (tacrolimus). Fujisawa, Deerfield, IL. | ||||
| 65 | Product Information. Oxbryta (voxelotor). Global Blood Therapeutics, Inc., South San Francisco, CA. | ||||
| 66 | DSouza DL, Levasseur LM, Nezamis J, Robbins DK, Simms L, Koch KM "Effect of alosetron on the pharmacokinetics of alprazolam." J Clin Pharmacol 41 (2001): 452-4. [PMID: 11304902] | ||||
| 67 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
| 68 | Product Information. Zanaflex (tizanidine). Acorda Therapeutics, Hawthorne, NY. | ||||
