Details of the Drug
General Information of Drug (ID: DMUM7HZ)
| Drug Name |
Fluorouracil
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
5-Fluorouracil; 51-21-8; fluorouracil; 5-FU; Fluoroplex; Adrucil; Efudex; Carac; Fluracil; Fluoroblastin; 5-fluoropyrimidine-2,4(1H,3H)-dione; Kecimeton; Timazin; Carzonal; Efudix; Arumel; Fluril; Queroplex; Fluracilum; Ulup; 5-Fluoracil; Phthoruracil; Fluro Uracil; 5-Fluoro-2,4(1H,3H)-pyrimidinedione; Ftoruracil; Fluorouracilum; Efurix; Fluri; 5 Fluorouracil; Effluderm (free base); 5-fluoro-1H-pyrimidine-2,4-dione; Fluorouracilo; Fluroblastin; Phtoruracil; 2,4-Dihydroxy-5-fluoropyrimidine; 2,4(1H,3H)-Pyrimidinedione, 5-fluoro-; Adrucil; Effluderm; Fluorouracile; Fluoruracil; Fluracedyl; Flurodex; Neofluor; Onkofluor; Ribofluor; Tetratogen; URF; Allergan Brand of Fluorouracil; Biosyn Brand of Fluorouracil; CSP Brand of Fluorouracil; Cinco FU; Dakota Brand of Fluorouracil; Dermatech Brand of Fluorouracil; Dermik Brandof Fluorouracil; Ferrer Brand of Fluorouracil; Fluoro Uracile ICN; Fluorouracil GRY; Fluorouracil Mononitrate; Fluorouracil Monopotassium Salt; Fluorouracil Monosodium Salt; Fluorouracil Potassium Salt; Fluorouracil Teva Brand; Fluorouracile Dakota; Fluorouracile [DCIT]; Fluorouracilo Ferrer Far; Gry Brand of Fluorouracil; Haemato Brand of Fluorouracil; Haemato fu; Hexal Brand of Fluorouracil; ICN Brand of Fluorouracil; Inhibits thymilidate synthetase; Medac Brand of Fluorouracil; Neocorp Brand of Fluorouracil; Onkoworks Brand of Fluorouracil; Ribosepharm Brand of Fluorouracil; Riemser Brand of Fluorouracil; Roche Brand of Fluorouracil; Teva Brand of Fluorouracil; F 6627; F0151; IN1335; U 8953; Adrucil (TN); Carac (TN); Dakota, Fluorouracile; Efudex (TN); Fluoro-Uracile ICN; Fluoro-uracile; Fluoro-uracilo; Fluoroplex (TN); Fluorouracil-GRY; Fluorouracilo [INN-Spanish]; Fluorouracilum [INN-Latin]; Haemato-fu; Ro 2-9757; U-8953; Ro-2-9757; Fluorouracil (JP15/USP/INN); Fluorouracil [USAN:INN:BAN:JAN]; 1-fluoro-1h-pyrimidine-2,4-dione; 2,4-Dioxo-5-fluoropryimidine; 2,4-Dioxo-5-fluoropyrimidine; 5 FU Lederle; 5 FU medac; 5 Fluorouracil biosyn; 5 HU Hexal; 5-FU (TN); 5-FU Lederle; 5-FU medac; 5-Faracil; 5-Fluor-2,4(1H,3H)-pyrimidindion; 5-Fluor-2,4(1H,3H)-pyrimidindion [Czech]; 5-Fluor-2,4-dihydroxypyrimidin; 5-Fluor-2,4-dihydroxypyrimidin [Czech]; 5-Fluor-2,4-pyrimidindiol; 5-Fluor-2,4-pyrimidindiol [Czech]; 5-Fluoracil [German]; 5-Fluoracyl; 5-Fluoro-2,4-pyrimidinedione; 5-Fluoropyrimidin-2,4-diol; 5-Fluoropyrimidine-2,4-dione; 5-Fluorouracil-biosyn; 5-Fluoruracil; 5-Fluoruracil [German]; 5-Ftouracyl; 5-HU Hexal; 5-fluoro uracil; 5FU
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Immunosuppressive Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
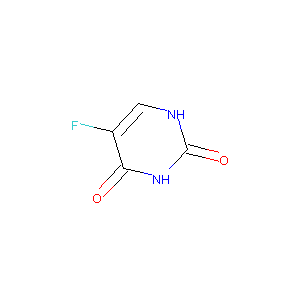 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 130.08 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Experimental Cancer Drug Sensitivity Information
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Fluorouracil
Coadministration of a Drug Treating the Disease Different from Fluorouracil (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
| DIG |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pharmaceutical Formulation |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
| 1 | Fluorouracil FDA Label | ||||
|---|---|---|---|---|---|
| 2 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | ||||
| 3 | The ChEMBL database in 2017. Nucleic Acids Res. 2017 Jan 4;45(D1):D945-D954. | ||||
| 4 | Design and development of antisense drugs. Expert Opin. Drug Discov. 2008 3(10):1189-1207. | ||||
| 5 | BDDCS applied to over 900 drugs | ||||
| 6 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 7 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 8 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 9 | Genome-wide association study of chemotherapeutic agent-induced severe neutropenia/leucopenia for patients in Biobank Japan. Cancer Sci. 2013 Aug;104(8):1074-82. doi: 10.1111/cas.12186. Epub 2013 Jun 10. | ||||
| 10 | The efficacy of the combination therapy of 5-fluorouracil, cisplatin and leucovorin for hepatocellular carcinoma and its predictable factors. Cancer Chemother Pharmacol. 2004 Apr;53(4):296-304. | ||||
| 11 | Enhancement of the antitumour activity of 5-fluorouracil (5-FU) by inhibiting dihydropyrimidine dehydrogenase activity (DPD) using 5-chloro-2,4-dihydroxypyridine (CDHP) in human tumour cells. Eur J Cancer. 2002 Jun;38(9):1271-7. | ||||
| 12 | Human equilibrative nucleoside transporter 1, as a predictor of 5-fluorouracil resistance in human pancreatic cancer. Anticancer Res. 2007 Jul-Aug;27(4B):2241-9. | ||||
| 13 | ATP-binding cassette C transporters in human pancreatic carcinoma cell lines. Upregulation in 5-fluorouracil-resistant cells. Pancreatology. 2009;9(1-2):136-44. | ||||
| 14 | Transport mechanism and substrate specificity of human organic anion transporter 2 (hOat2 [SLC22A7]). J Pharm Pharmacol. 2005 May;57(5):573-8. | ||||
| 15 | Enhancing chemosensitivity in oral squamous cell carcinoma by lentivirus vector-mediated RNA interference targeting EGFR and MRP2. Oncol Lett. 2016 Sep;12(3):2107-2114. | ||||
| 16 | Role of BCRP as a biomarker for predicting resistance to 5-fluorouracil in breast cancer. Cancer Chemother Pharmacol. 2009 May;63(6):1103-10. | ||||
| 17 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | ||||
| 18 | Roles of cytochromes P450 1A2, 2A6, and 2C8 in 5-fluorouracil formation from tegafur, an anticancer prodrug, in human liver microsomes. Drug Metab Dispos. 2000 Dec;28(12):1457-63. | ||||
| 19 | 5-Fluorouracil pharmacogenomics: still rocking after all these years? Pharmacogenomics. 2011 Feb;12(2):251-65. | ||||
| 20 | Uridine phosphorylase in breast cancer: a new prognostic factor? Front Biosci. 2006 Sep 1;11:2759-66. | ||||
| 21 | 5-Fluorouracil toxicity-attributable IVS14 + 1G > A mutation of the dihydropyrimidine dehydrogenase gene in Polish colorectal cancer patients. Pharmacol Rep. 2008 Mar-Apr;60(2):238-42. | ||||
| 22 | Orotate phosphoribosyltransferase is overexpressed in malignant pleural mesothelioma: Dramatically responds one case in high OPRT expression. Rare Dis. 2016 Apr 5;4(1):e1165909. | ||||
| 23 | Combined suicide gene therapy for human colon cancer cells using adenovirus-mediated transfer of escherichia coli cytosine deaminase gene and Escherichia coli uracil phosphoribosyltransferase gene with 5-fluorocytosine. Cancer Gene Ther. 2000 Jul;7(7):1015-22. | ||||
| 24 | PharmGKB: A worldwide resource for pharmacogenomic information. Wiley Interdiscip Rev Syst Biol Med. 2018 Jul;10(4):e1417. (ID: PA150653776) | ||||
| 25 | Antitumour effects on human colorectal carcinomas cells by stable silencing of phospholipase C-gamma 1 with lentivirus-delivered siRNA. Chin Med J (Engl). 2007 May 5;120(9):749-54. | ||||
| 26 | 5-Fluorouracil: identification of novel downstream mediators of tumour response. Anticancer Res. 2004 Mar-Apr;24(2A):417-23. | ||||
| 27 | Cellular response to 5-fluorouracil (5-FU) in 5-FU-resistant colon cancer cell lines during treatment and recovery. Mol Cancer. 2006 May 18;5:20. doi: 10.1186/1476-4598-5-20. | ||||
| 28 | Arsenic trioxide reverses the chemoresistance in hepatocellular carcinoma: a targeted intervention of 14-3-3/NF-B feedback loop. J Exp Clin Cancer Res. 2018 Dec 20;37(1):321. doi: 10.1186/s13046-018-1005-y. | ||||
| 29 | Transcriptional profiling of MCF7 breast cancer cells in response to 5-Fluorouracil: relationship with cell cycle changes and apoptosis, and identification of novel targets of p53. Int J Cancer. 2006 Sep 1;119(5):1164-75. | ||||
| 30 | Multi-level gene expression profiles affected by thymidylate synthase and 5-fluorouracil in colon cancer. BMC Genomics. 2006 Apr 3;7:68. doi: 10.1186/1471-2164-7-68. | ||||
| 31 | New insights into the mechanisms underlying 5-fluorouracil-induced intestinal toxicity based on transcriptomic and metabolomic responses in human intestinal organoids. Arch Toxicol. 2021 Aug;95(8):2691-2718. doi: 10.1007/s00204-021-03092-2. Epub 2021 Jun 20. | ||||
| 32 | Zhou-Pan XR, Seree E, Zhou XJ, et al "Involvement of human liver cytochrome P450 3A in vinblastine metabolism: drug interactions." Cancer Res 53 (1993): 5121-6. [PMID: 8221648] | ||||
| 33 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 34 | Johnson EJ, MacGowan AP, Potter MN, et al "Reduced absorption of oral ciprofloxacin after chemotherapy for haematological malignancy." J Antimicrob Chemother 25 (1990): 837-42. [PMID: 2373666] | ||||
| 35 | Product Information. Arava (leflunomide). Hoechst Marion-Roussel Inc, Kansas City, MO. | ||||
| 36 | Product Information. Prolia (denosumab). Amgen USA, Thousand Oaks, CA. | ||||
| 37 | Blakely KM, Drucker AM, Rosen CF "Drug-induced photosensitivity-an update: Culprit drugs, prevention and management." Drug Saf 42 (2019): 827-47. [PMID: 30888626] | ||||
| 38 | Bennett CL, Nebeker JR, Samore MH, et al "The Research on Adverse Drug Events and Reports (RADAR) project." JAMA 293 (2005): 2131-40. [PMID: 15870417] | ||||
| 39 | Product Information. Vumerity (diroximel fumarate). Alkermes, Inc, Cambridge, MA. | ||||
| 40 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 41 | Product Information. Gilenya (fingolimod). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 42 | Product Information. Ocrevus (ocrelizumab). Genentech, South San Francisco, CA. | ||||
| 43 | Product Information. Synribo (omacetaxine). Teva Pharmaceuticals USA, North Wales, PA. | ||||
| 44 | Product Information. Arcalyst (rilonacept). Regeneron Pharmaceuticals Inc, Tarrytown, NY. | ||||
| 45 | Product Information. Cimzia (certolizumab). UCB Pharma Inc, Smyrna, GA. | ||||
| 46 | CDC. Centers for Disease Control and Prevention/ "Recommendations of the advisory committtee on immunization practices (ACIP): use of vaccines and immune globulins in persons with altered immunocompetence." MMWR Morb Mortal Wkly Rep 42(RR-04) (1993): 1-18. [PMID: 20300058] | ||||
