Details of the Drug
General Information of Drug (ID: DMUNTE3)
| Drug Name |
Estradiol
|
|||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Aerodiol; Alora; Altrad; Aquadiol; Bardiol; Climaderm; Climara; Compudose; Corpagen; Dermestril; Destradiol; Dihydrofolliculin; Dihydromenformon; Dihydrotheelin; Dihydroxyesterin; Dihydroxyestrin; Dihydroxyoestrin; Dimenformon; Diogyn; Diogynets; Divigel; Elestrin; Encore; Esclim; Estrace; Estraderm; Estradiolo; Estradiolum; Estradot; Estraldine; Estrasorb; Estreva; Estrifam; Estring; Estroclim; Estrodiolum; Estrogel; Estrovite; Evamist; Evorel; Extrasorb; Femanest; Femestral; Femestrol; Fempatch; Femtran; Follicyclin; Gelestra; Ginedisc; Ginosedol; GynPolar; Gynergon; Gynestrel; Gynodiol; Gynoestryl; Innofem; Lamdiol; Macrodiol; Macrol; Menest; Menorest; Microdiol; Nordicol; Oesclim; Oestergon; Oestradiol; Oestradiolum; Oestrogel; Oestroglandol; Oestrogynal; Ovahormon; Ovasterol; Ovastevol; Ovociclina; Ovocyclin; Ovocycline; Ovocylin; Perlatanol; Polyestradiol; Primofol; Profoliol; Progynon; Syndiol; Systen; Tradelia; Trocosone; Vagifem; Vivelle; Zerella; Zesteem; Zesteen; Zumenon; Climara Forte; Component of Menrium; Estraderm MX; Estraderm TTS; Estradiolo [DCIT]; Estradiolum [INN]; Estring vaginal ring; Estrofem Forte; Oestradiol Berco; Oestradiol R; Progynon DH; Sandrena Gel; Sisare Gel; Trial SAT; CMC_11154; Compudose 200; Compudose 365; E 2; E 8875; E0025; Epiestriol 50; Estraderm TTS 100; Estraderm TTS 50; Estrapak 50; Estroclim 50; Estrofem 2; Sandrena 1; [3H]]estradiol; Activella (TN); Alora (TN); Alpha-Oestradiol; AngeliQ (TN); B-Estradiol; Beta-Estradiol; Beta-Oestradiol; Beta-estradiol; Cis-Estradiol; Cis-Oestradiol; Climara (TN); D-Estradiol; D-Oestradiol; Divigel (TN); E(sub 2); Elestrin (TN); Estrace (TN); Estraderm (TN); Estraderm TTS (TN); Estradiol [USAN:INN]; Estradiol acetate (TN); Estradiol cypionate (TN); Estradiol valerate (TN); Estradiol-17 beta; Estradiol-17beta; Estrasorb (TN); Estrasorb Topical (TN); Estring (TN); Estrofem (TN); Estrogel (TN); EvaMist (TN); Femring (TN); Innofem (TN); Menostar (TN); Oestradiol-17beta; Progynon-DH; Progynova (TN); S-21400; SK-Estrogens; SL-1100; VIVELLE-DOT; Vagifem (TN); Vivelle (TN); [3H]-estradiol; Estradiol-17-beta; Estradiol-3,17beta; Oestradiol-17-beta; Vivelle-Dot (TN); D-3,17beta-Estradiol; [2,4,6,7-3H]-E2; 17 beta-Estradiol; 17-.BETA.-Estradiol; 17-beta-OH-estradiol; 17-beta-estradiol; 17.beta.-Estradiol; 17.beta.-Oestradiol; 17b-Oestradiol; 17beta oestradiol; 17beta-Estradiol; 17beta-Oestradiol; 3,17-beta-Estradiol; 3,17-beta-Oestradiol; 3,17.beta.-Estradiol; 3,17beta-Estradiol
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Estrogens
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
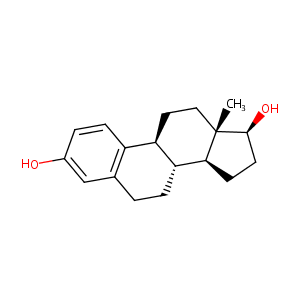 |
|||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 272.4 | ||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4 | |||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | |||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Acne vulgaris | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | ED80 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Estradiol
Coadministration of a Drug Treating the Disease Different from Estradiol (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Estradiol FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1013). | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Pharmacokinetic and pharmacologic variation between different estrogen products. J Clin Pharmacol. 1995 Sep;35(9S):18S-24S. doi: 10.1002/j.1552-4604.1995.tb04143.x. | ||||
| 5 | Tubic-Grozdanis M, Hilfinger JM, Amidon GL, Kim JS, Kijek P, Staubach P, Langguth P: Pharmacokinetics of the CYP 3A substrate simvastatin following administration of delayed versus immediate release oral dosage forms. Pharm Res. 2008 Jul;25(7):1591-600. doi: 10.1007/s11095-007-9519-6. Epub 2008 Jan 24. | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 8 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 9 | Reprint of Are all estrogens the same Maturitas. 2008 Sep-Oct;61(1-2):195-201. | ||||
| 10 | Sterol transport by the human breast cancer resistance protein (ABCG2) expressed in Lactococcus lactis. J Biol Chem. 2003 Jun 6;278(23):20645-51. | ||||
| 11 | Antiestrogens and steroid hormones: substrates of the human P-glycoprotein. Biochem Pharmacol. 1994 Jul 19;48(2):287-92. | ||||
| 12 | Isoform-specific regulation of cytochromes P450 expression by estradiol and progesterone. Drug Metab Dispos. 2013 Feb;41(2):263-9. | ||||
| 13 | Characterization of the oxidative metabolites of 17beta-estradiol and estrone formed by 15 selectively expressed human cytochrome p450 isoforms. Endocrinology. 2003 Aug;144(8):3382-98. | ||||
| 14 | Cytochrome P450 isoforms catalyze formation of catechol estrogen quinones that react with DNA. Metabolism. 2007 Jul;56(7):887-94. | ||||
| 15 | Functional characterization of human and cynomolgus monkey UDP-glucuronosyltransferase 1A1 enzymes. Life Sci. 2010 Aug 14;87(7-8):261-8. | ||||
| 16 | CYP2C19*17 is associated with decreased breast cancer risk. Breast Cancer Res Treat. 2009 May;115(2):391-6. | ||||
| 17 | Cytochrome P450 1A2 (CYP1A2) activity and risk factors for breast cancer: a cross-sectional study. Breast Cancer Res. 2004;6(4):R352-65. | ||||
| 18 | Drug Interactions Flockhart Table | ||||
| 19 | Role of cytochrome P450 2C8 in drug metabolism and interactions. Pharmacol Rev. 2016 Jan;68(1):168-241. | ||||
| 20 | Proposed role of the sulfotransferase/sulfatase pathway in modulating yolk steroid effects. Integr Comp Biol. 2008 Sep;48(3):419-27. | ||||
| 21 | Influence of estradiol-17 beta and progesterone on catechol-O-methyltransferase and monoamine oxidase activities in uterine artery and myometrium of ovariectomized pigs. Arch Vet Pol. 1993;33(1-2):29-37. | ||||
| 22 | The metabolism of 17 beta-estradiol by lactoperoxidase: a possible source of oxidative stress in breast cancer. Carcinogenesis. 1994 Nov;15(11):2637-43. | ||||
| 23 | Aldo-keto Reductase 1B15 (AKR1B15): a mitochondrial human aldo-keto reductase with activity toward steroids and 3-keto-acyl-CoA conjugates. J Biol Chem. 2015 Mar 6;290(10):6531-45. | ||||
| 24 | Expression patterns of 17beta-hydroxysteroid dehydrogenase 14 in human tissues. Horm Metab Res. 2012 Dec;44(13):949-56. | ||||
| 25 | Isolation and characterization of the UGT2B28 cDNA encoding a novel human steroid conjugating UDP-glucuronosyltransferase. Biochemistry. 2001 Apr 3;40(13):3869-81. | ||||
| 26 | 17-Estradiol Activates HSF1 via MAPK Signaling in ER-Positive Breast Cancer Cells. Cancers (Basel). 2019 Oct 11;11(10):1533. doi: 10.3390/cancers11101533. | ||||
| 27 | Comparison of HepG2 and HepaRG by whole-genome gene expression analysis for the purpose of chemical hazard identification. Toxicol Sci. 2010 May;115(1):66-79. | ||||
| 28 | Comparison of phenotypic and transcriptomic effects of false-positive genotoxins, true genotoxins and non-genotoxins using HepG2 cells. Mutagenesis. 2011 Sep;26(5):593-604. | ||||
| 29 | Genistein and bisphenol A exposure cause estrogen receptor 1 to bind thousands of sites in a cell type-specific manner. Genome Res. 2012 Nov;22(11):2153-62. | ||||
| 30 | Persistent and non-persistent changes in gene expression result from long-term estrogen exposure of MCF-7 breast cancer cells. J Steroid Biochem Mol Biol. 2011 Feb;123(3-5):140-50. | ||||
| 31 | Product Information. Tykerb (lapatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 32 | Product Information. Tukysa (tucatinib). Seattle Genetics Inc, Bothell, WA. | ||||
| 33 | Baciewicz AM "Oral contraceptive drug interactions." Ther Drug Monit 7 (1985): 26-35. [PMID: 2859674] | ||||
| 34 | Product Information. Ambien (zolpidem). sanofi-aventis, Bridgewater, NJ. | ||||
| 35 | Back DJ, Breckenridge AM, Crawford FE, MacIver M, Orne ML, Rowe PH "Interindividual variation and drug interactions with hormonal steroid contraceptives." Drugs 21 (1981): 46-61. [PMID: 7009137] | ||||
| 36 | Devenport MH, Crook D, Wynn V, Lees LJ "Metabolic effects of low-dose fluconazole in healthy female users and non-users of oral contraceptives." Br J Clin Pharmacol 27 (1989): 851-9. [PMID: 2547410] | ||||
| 37 | Gardner MJ, Tornatore KM, Jusko WJ, Kanarkowski R "Effects of tobacco smoking and oral contraceptive use on theophylline disposition." Br J Clin Pharmacol 16 (1983): 271-80. [PMID: 6626419] | ||||
| 38 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 39 | Product Information. Ketek (telithromycin). Aventis Pharmaceuticals, Bridgewater, NJ. | ||||
| 40 | Product Information. Lipitor (atorvastatin). Parke-Davis, Morris Plains, NJ. | ||||
| 41 | Notelovitz M "Oral contraception and coagulation." Clin Obstet Gynecol 28 (1985): 73-83. [PMID: 3987135] | ||||
| 42 | Product Information. Kalydeco (ivacaftor). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 43 | Adson DE, Kotlyar M "A probable interaction between a very low-dose oral contraceptive and the antidepressant nefazodone: a case report." J Clin Psychopharmacol 21 (2001): 618-9. [PMID: 11763013] | ||||
| 44 | EMEA. European Medicines Agency "EPARs. European Union Public Assessment Reports.". | ||||
| 45 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 46 | Product Information. Dantrium (dantrolene). Procter and Gamble Pharmaceutic, Cincinnati, OH. | ||||
| 47 | Product Information. Tazverik (tazemetostat). Epizyme, Inc, Cambridge, MA. | ||||
| 48 | Product Information. Victrelis (boceprevir). Schering-Plough Corporation, Kenilworth, NJ. | ||||
| 49 | Product Information. Norvir (ritonavir). Abbott Pharmaceutical, Abbott Park, IL. | ||||
| 50 | Product Information. Incivek (telaprevir). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 51 | Product Information. Aptivus (tipranavir). Boehringer-Ingelheim, Ridgefield, CT. | ||||
| 52 | Product Information. Fortovase (saquinavir) Roche Laboratories, Nutley, NJ. | ||||
| 53 | Product Information. Prezista (darunavir). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 54 | Product Information. Vaprisol (conivaptan). Cumberland Pharmaceuticals Inc, Nashville, TN. | ||||
| 55 | Product Information. Orladeyo (berotralstat). BioCryst Pharmaceuticals Inc, Durham, NC. | ||||
| 56 | Product Information. Alunbrig (brigatinib). Ariad Pharmaceuticals Inc, Cambridge, MA. | ||||
| 57 | Product Information. Copiktra (duvelisib). Verastem, Inc., Needham, MA. | ||||
| 58 | Product Information. Exjade (deferasirox). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 59 | Product Information. Tasigna (nilotinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 60 | Product Information. Sprycel (dasatinib). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 61 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 62 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 63 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 64 | Product Information. Zokinvy (lonafarnib). Eiger BioPharmaceuticals, Palo Alto, CA. | ||||
| 65 | Product Information. Prograf (tacrolimus). Fujisawa, Deerfield, IL. | ||||
| 66 | Product Information. Oxbryta (voxelotor). Global Blood Therapeutics, Inc., South San Francisco, CA. | ||||
| 67 | Doherty MM, Charman WN "The mucosa of the small intestine: how clinically relevant as an organ of drug metabolism?" Clin Pharmacokinet 41 (2002): 235-53. [PMID: 11978143] | ||||
| 68 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
