Details of the Drug
General Information of Drug (ID: DMUY35B)
| Drug Name |
Progesterone
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
progesterone; 57-83-0; Agolutin; Pregn-4-ene-3,20-dione; Luteohormone; Crinone; 4-Pregnene-3,20-dione; Utrogestan; Syngesterone; Progestin; Luteol; Corpus luteum hormone; Progesterol; Progesteronum; Pregnenedione; Glanducorpin; Prometrium; Corlutin; Cyclogest; Progestron; Gestormone; Progestone; Gestone; Progestasert; Progestronol; Methylpregnone; Hormoflaveine; Syngestrets; Proluton; Progekan; Nalutron; Lutoform; Gynlutin; Gesterol; Fologenon; Corporin; Corlutina; Syntolutan; Prolidon; Membrettes; Lutocyclin; Luteodyn; Lucorteum; Corluvite; LPCN-1107; Progesterone (oral, preterm labor); Progesterone (oral, preterm labor), Lipocine
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
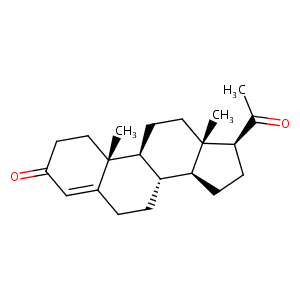 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 314.5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Progesterone (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (ANDA) 075906. | ||||
|---|---|---|---|---|---|
| 2 | Progesterone FDA Label | ||||
| 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2377). | ||||
| 4 | ClinicalTrials.gov (NCT00822900) Progesterone for the Treatment of Traumatic Brain Injury III. U.S. National Institutes of Health. | ||||
| 5 | ClinicalTrials.gov (NCT04365127) Progesterone for the Treatment of COVID-19 in Hospitalized Men. U.S. National Institutes of Health. | ||||
| 6 | BDDCS applied to over 900 drugs | ||||
| 7 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 8 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 9 | Clinical pipeline report, company report or official report of lipocine. | ||||
| 10 | Sterol transport by the human breast cancer resistance protein (ABCG2) expressed in Lactococcus lactis. J Biol Chem. 2003 Jun 6;278(23):20645-51. | ||||
| 11 | Comparative studies on in vitro methods for evaluating in vivo function of MDR1 P-glycoprotein. Pharm Res. 2001 Dec;18(12):1660-8. | ||||
| 12 | Contribution of human hepatic cytochrome P450 isoforms to regioselective hydroxylation of steroid hormones. Xenobiotica. 1998 Jun;28(6):539-47. | ||||
| 13 | Effect of genetic polymorphism on the metabolism of endogenous neuroactive substances, progesterone and p-tyramine, catalyzed by CYP2D6. Brain Res Mol Brain Res. 2004 Oct 22;129(1-2):117-23. | ||||
| 14 | Progesterone and testosterone hydroxylation by cytochromes P450 2C19, 2C9, and 3A4 in human liver microsomes. Arch Biochem Biophys. 1997 Oct 1;346(1):161-9. | ||||
| 15 | Allelic variants of human cytochrome P450 1A1 (CYP1A1): effect of T461N and I462V substitutions on steroid hydroxylase specificity. Pharmacogenetics. 2000 Aug;10(6):519-30. | ||||
| 16 | Human glutathione transferase A3-3, a highly efficient catalyst of double-bond isomerization in the biosynthetic pathway of steroid hormones. J Biol Chem. 2001 Aug 31;276(35):33061-5. | ||||
| 17 | Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002 Feb-May;34(1-2):83-448. | ||||
| 18 | Human prostate CYP3A5: identification of a unique 5'-untranslated sequence and characterization of purified recombinant protein. Biochem Biophys Res Commun. 1999 Jul 14;260(3):676-81. | ||||
| 19 | Catalytic properties of polymorphic human cytochrome P450 1B1 variants. Carcinogenesis. 1999 Aug;20(8):1607-13. | ||||
| 20 | The role of cytochrome P450 3A (CYP3A) isoform(s) in oxidative metabolism of testosterone and benzphetamine in human adult and fetal liver. J Steroid Biochem Mol Biol. 1993 Jan;44(1):61-7. | ||||
| 21 | Progestagenic effects of tibolone are target gene-specific in human endometrial cells. J Soc Gynecol Investig. 2006 Sep;13(6):459-65. | ||||
| 22 | Steroid hormone hydroxylase specificities of eleven cDNA-expressed human cytochrome P450s. Arch Biochem Biophys. 1991 Oct;290(1):160-6. | ||||
| 23 | Broad substrate specificity of human cytochrome P450 46A1 which initiates cholesterol degradation in the brain. Biochemistry. 2003 Dec 9;42(48):14284-92. | ||||
| 24 | Human 3-alpha hydroxysteroid dehydrogenase type 3 (3alpha-HSD3): the V54L mutation restricting the steroid alternative binding and enhancing the 20alpha-HSD activity. J Steroid Biochem Mol Biol. 2014 May;141:135-43. | ||||
| 25 | Human cytochrome P450 21A2, the major steroid 21-hydroxylase: structure of the enzyme progesterone substrate complex and rate-limiting C-H bond cleavage. J Biol Chem. 2015 May 22;290(21):13128-43. | ||||
| 26 | Engineering of CYP106A2 for steroid 9alpha- and 6beta-hydroxylation. Steroids. 2017 Apr;120:41-48. | ||||
| 27 | acillus megaterium SF185 spores exert protective effects against oxidative stress in vivo and in vitro. Sci Rep. 2019 Aug 19;9(1):12082. | ||||
| 28 | A Novel NADPH-dependent flavoprotein reductase from Bacillus megaterium acts as an efficient cytochrome P450 reductase. J Biotechnol. 2016 Aug 10;231:83-94. | ||||
| 29 | Unique transcriptome, pathways, and networks in the human endometrial fibroblast response to progesterone in endometriosis. Biol Reprod. 2011 Apr;84(4):801-15. | ||||
| 30 | Effects of progesterone treatment on expression of genes involved in uterine quiescence. Reprod Sci. 2011 Aug;18(8):781-97. | ||||
| 31 | Effect of the interaction between lipoxygenase pathway and progesterone on the regulation of hydroxysteroid 11-Beta dehydrogenase 2 in cultured human term placental trophoblasts. Biol Reprod. 2008 Mar;78(3):514-20. | ||||
| 32 | Progestin effects on expression of AKR1C1-AKR1C3, SRD5A1 and PGR in the Z-12 endometriotic epithelial cell line. Chem Biol Interact. 2013 Feb 25;202(1-3):218-25. | ||||
| 33 | Progesterone promotes differentiation of human cord blood fetal T cells into T regulatory cells but suppresses their differentiation into Th17 cells. J Immunol. 2011 Aug 15;187(4):1778-87. doi: 10.4049/jimmunol.1003919. Epub 2011 Jul 18. | ||||
| 34 | Progesterone regulation of implantation-related genes: new insights into the role of oestrogen. Cell Mol Life Sci. 2007 Apr;64(7-8):1009-32. | ||||
| 35 | Elucidating progesterone effects in breast cancer: cross talk with PDGF signaling pathway in smooth muscle cell. J Cell Biochem. 2007 Jan 1;100(1):174-83. doi: 10.1002/jcb.21045. | ||||
| 36 | Steroid signalling in the ovarian surface epithelium. Trends Endocrinol Metab. 2005 Sep;16(7):327-33. | ||||
| 37 | Baciewicz AM "Oral contraceptive drug interactions." Ther Drug Monit 7 (1985): 26-35. [PMID: 2859674] | ||||
| 38 | Multum Information Services, Inc. Expert Review Panel. | ||||
| 39 | Product Information. Ambien (zolpidem). sanofi-aventis, Bridgewater, NJ. | ||||
| 40 | Back DJ, Breckenridge AM, Crawford FE, MacIver M, Orne ML, Rowe PH "Interindividual variation and drug interactions with hormonal steroid contraceptives." Drugs 21 (1981): 46-61. [PMID: 7009137] | ||||
| 41 | Devenport MH, Crook D, Wynn V, Lees LJ "Metabolic effects of low-dose fluconazole in healthy female users and non-users of oral contraceptives." Br J Clin Pharmacol 27 (1989): 851-9. [PMID: 2547410] | ||||
| 42 | Product Information. Synercid (dalfopristin-quinupristin) Rhone-Poulenc Rorer, Collegeville, PA. | ||||
| 43 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 44 | Product Information. Ketek (telithromycin). Aventis Pharmaceuticals, Bridgewater, NJ. | ||||
| 45 | Product Information. Tykerb (lapatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 46 | Product Information. Lipitor (atorvastatin). Parke-Davis, Morris Plains, NJ. | ||||
| 47 | Product Information. Emend (aprepitant). Merck & Company Inc, West Point, PA. | ||||
| 48 | Product Information. Serzone (nefazodone). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 49 | EMEA. European Medicines Agency "EPARs. European Union Public Assessment Reports.". | ||||
| 50 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 51 | Product Information. Tazverik (tazemetostat). Epizyme, Inc, Cambridge, MA. | ||||
| 52 | Product Information. Victrelis (boceprevir). Schering-Plough Corporation, Kenilworth, NJ. | ||||
| 53 | Product Information. Incivek (telaprevir). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 54 | Product Information. Adcetris (brentuximab vedotin). Seattle Genetics Inc, Bothell, WA. | ||||
| 55 | Barry M, Mulcahy F, Merry C, Gibbons S, Back D "Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat patients with HIV infection." Clin Pharmacokinet 36 (1999): 289-304. [PMID: 10320951] | ||||
| 56 | Product Information. Fortovase (saquinavir) Roche Laboratories, Nutley, NJ. | ||||
| 57 | Product Information. Prezista (darunavir). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 58 | Product Information. Vaprisol (conivaptan). Cumberland Pharmaceuticals Inc, Nashville, TN. | ||||
| 59 | Product Information. Orladeyo (berotralstat). BioCryst Pharmaceuticals Inc, Durham, NC. | ||||
| 60 | Product Information. Alunbrig (brigatinib). Ariad Pharmaceuticals Inc, Cambridge, MA. | ||||
| 61 | Product Information. Copiktra (duvelisib). Verastem, Inc., Needham, MA. | ||||
| 62 | Product Information. Exjade (deferasirox). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 63 | Product Information. Tasigna (nilotinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 64 | Product Information. Gleevec (imatinib mesylate). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 65 | Product Information. Sprycel (dasatinib). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 66 | Doherty MM, Charman WN "The mucosa of the small intestine: how clinically relevant as an organ of drug metabolism?" Clin Pharmacokinet 41 (2002): 235-53. [PMID: 11978143] | ||||
| 67 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 68 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 69 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 70 | Product Information. Zokinvy (lonafarnib). Eiger BioPharmaceuticals, Palo Alto, CA. | ||||
| 71 | Product Information. Prograf (tacrolimus). Fujisawa, Deerfield, IL. | ||||
| 72 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
