Details of the Drug
General Information of Drug (ID: DMUIE76)
| Drug Name |
Acetaminophen
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
acetaminophen; 4-Acetamidophenol; Paracetamol; 103-90-2; Tylenol; N-(4-Hydroxyphenyl)acetamide; APAP; Acetaminofen; Panadol; Datril; p-Hydroxyacetanilide; N-Acetyl-p-aminophenol; p-Acetamidophenol; Algotropyl; Naprinol; Lonarid; 4'-Hydroxyacetanilide; Multin; Acenol; Acamol; Anelix; p-Acetaminophenol; Liquagesic; Acetagesic; Gelocatil; Servigesic; Acetalgin; Abensanil; Pyrinazine; Injectapap; Clixodyne; Valgesic; Tussapap; Finimal; Paracet; Homoolan; Febrolin; Febrilix; Febridol; Dymadon; Anaflon; Apamide; Valadol; Tralgon; Tabalgin; Lestemp; Alvedon; Abenol; Abrol; Abrolet; Acephen; Acertol; Acetaco; Acetamol; Acetavance; Acetofen; Actamin; Actimol; Afebrin; Afebryl; Aferadol; Algesidal; Algina; Algomol; Alpiny; Alpinyl; Amadil; Aminofen; Analter; Anapap; Andox; Anhiba; Antidol; Anuphen; Apacet; Apadon; Apitrelal; Arfen; Arthralgen; Asetam; Asomal; Aspac; Asplin; Atasol; Atralidon; Babikan; Bacetamol; Banesin; Benmyo; Biocetamol; Cadafen; Calapol; Calmanticold; Calonal; Calpol; Capital; Captin; Causalon; Cefalex; Cetadol; Codabrol; Codalgin; Codapane; Codicet; Codisal; Codoliprane; Cofamol; Conacetol; Cosutone; Cuponol; Curadon; Curpol; Custodial; Dafalgan; Darocet; Darvocet; Daygrip; Deminofen; Democyl; Demogripal; Desfebre; Dhamol; Dimindol; Dirox; Disprol; Dolcor; Dolefin; Dolegrippin; Dolgesic; Doliprane; Dolko; Dolofugin; Doloreduct; Dolorfug; Dolorstop; Dolotec; Dolprone; Dorocoff; Dresan; Dristancito; Duaneo; Dularin; Duorol; Duracetamol; Durapan; Dypap; Ecosetol; Elixodyne; Empracet; Enelfa; Eneril; Excipain; Exdol; Fanalgic; Farmadol; Febranine; Febrectal; Febrectol; Febrex; Febricet; Febrin; Febrinol; Fendon; Fensum; Fepanil; Fevor; Finiweh; Fluparmol; Geluprane; Genapap; Genebs; Grippostad; Gynospasmine; Hedex; Ildamol; Inalgex; Infadrops; Janupap; Kataprin; Korum; Labamol; Lekadol; Lemgrip; Lemsip; Liqiprine; Lupocet; Lyteca; Magnidol; Malgis; Malidens; Maxadol; Medocodene; Mexalen; Minafen; Minoset; Miralgin; Momentum; NEBS; Napafen; Nealgyl; NeoCitran; Neodol; Neodolito; Neopap; Neotrend; Neuridon; NilnOcen; Nina; Nobedon; Nodolex; Noral; Ofirmev; Oltyl; Oralgan; Ortensan; Oxycocet; Paceco; Pacemo; Pacemol; Pacet; Pacimol; Paedialgon; Paedol; Painex; Paldesic; Pamol; Panacete; Panadeine; Panadiene; Panaleve; Panamax; Panasorb; Panasorbe; Panets; Panex; Panodil; Panofen; Pantalgin; Paracemol; Paracenol; Paracetamole; Paracetamolo; Paracetanol; Paracetol; Paracin; Paracod; Paracodol; Parador; Paradrops; Parakapton; Parake; Paralen; Paralief; Paralink; Paralyoc; Paramol; Paramolan; Paranox; Parapan; Parasedol; Parasin; Paraspen; Parcetol; Parelan; Parmol; Parogal; Paroma; Pasolind; Pediapirin; Pediatrix; Pedric; Perfalgan; Phendon; Phenipirin; Phogoglandin; Pinex; Piramin; Pirinasol; Plicet; Polmofen; Predimol; Predualito; Prodol; Prompt; Prontina; Puernol; Pulmofen; Pyrigesic; Pyromed; Redutemp; Reliv; Remedol; Rivalgyl; Robigesic; Rounox; RubieMol; Rubophen; Rupemol; Salzone; Sanicet; Sanicopyrine; Scanol; Scentalgyl; Schmerzex; Sedalito; Semolacin; Seskamol; Setakop; Setamol; Setol; Sifenol; Sinaspril; Sinedol; Sinmol; Stanback; Stopain; Sunetheton; Supofen; Suppap; TYL; Tachiprina; Tapanol; Tapar; Tazamol; Temlo; Tempanal; Tempra; Termacet; Termalgin; Termalgine; Termofren; Tiffy; Titralgan; Treuphadol; Tricoton; Tylex; Tylol; Tymol; Upsanol; Utragin; Valorin; Veralgina; Vermidon; Verpol; Vips; Viruflu; Vivimed; Volpan; Zatinol; Zolben; Aceta Elixir; Actifed Plus; Aspirin free anacin; Bayer Select; D oliprane; Dymadon Co; Fortalidon P; Gattaphen T; Gripin Bebe; Helon N; Influbene N; Jin Gang; Lonarid Mono; Lyteca Syrup; Malex N; Panadeine Co; Panale ve; Pasolind N; Spalt N; Supadol mono; Toximer P; Treupel N; Treupel mon; Ty lenol; Tylex CD; Anacin 3; A-Per; Accu-Tap; Ultracet
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Analgesics
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
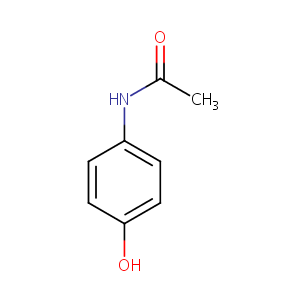 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 151.16 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Allergic rhinitis | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | CA08.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Acetaminophen
Coadministration of a Drug Treating the Disease Different from Acetaminophen (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Acetaminophen FDA Label | ||||
|---|---|---|---|---|---|
| 2 | The Diversion of Ultram, Ultracet, and generic tramadol HCL. J Addict Dis. 2006;25(2):53-8. | ||||
| 3 | PharmGKB summary: pathways of acetaminophen metabolism at the therapeutic versus toxic doses. Pharmacogenet Genomics. 2015 Aug;25(8):416-26. doi: 10.1097/FPC.0000000000000150. | ||||
| 4 | BDDCS applied to over 900 drugs | ||||
| 5 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | Bannwarth B, Pehourcq F: [Pharmacologic basis for using paracetamol: pharmacokinetic and pharmacodynamic issues]. Drugs. 2003;63 Spec No 2:5-13. | ||||
| 8 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 9 | Acetaminophen-NAPQI hepatotoxicity: a cell line model system genome-wide association study. Toxicol Sci. 2011 Mar;120(1):33-41. doi: 10.1093/toxsci/kfq375. Epub 2010 Dec 22. | ||||
| 10 | A study of HLA class I and class II 4-digit allele level in Stevens-Johnson syndrome and toxic epidermal necrolysis. Int J Immunogenet. 2011 Aug;38(4):303-9. doi: 10.1111/j.1744-313X.2011.01011.x. Epub 2011 May 4. | ||||
| 11 | Augmentation effect of combination therapy of aripiprazole and antidepressants on forced swimming test in mice. Psychopharmacology (Berl). 2009 Sep;206(1):97-107. | ||||
| 12 | Mechanism of action of paracetamol. Am J Ther. 2005 Jan-Feb;12(1):46-55. | ||||
| 13 | Effect of acetaminophen on expression and activity of rat liver multidrug resistance-associated protein 2 and P-glycoprotein. Biochem Pharmacol. 2004 Aug 15;68(4):791-8. | ||||
| 14 | Metabolic interactions between acetaminophen (paracetamol) and two flavonoids, luteolin and quercetin, through in-vitro inhibition studies. J Pharm Pharmacol. 2017 Dec;69(12):1762-1772. | ||||
| 15 | Acetaminophen induced acute liver failure via oxidative stress and JNK activation: protective role of taurine by the suppression of cytochrome P450 2E1. Free Radic Res. 2010 Mar;44(3):340-55. | ||||
| 16 | Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002 Feb-May;34(1-2):83-448. | ||||
| 17 | Preferred orientations in the binding of 4'-hydroxyacetanilide (acetaminophen) to cytochrome P450 1A1 and 2B1 isoforms as determined by 13C- and 15N-NMR relaxation studies. J Med Chem. 1994 Mar 18;37(6):860-7. | ||||
| 18 | Effect of UDP-glucuronosyltransferase (UGT) 1A polymorphism (rs8330 and rs10929303) on glucuronidation status of acetaminophen. Dose Response. 2017 Sep 11;15(3):1559325817723731. | ||||
| 19 | Retinoid X receptor alpha regulates the expression of glutathione s-transferase genes and modulates acetaminophen-glutathione conjugation in mouse liver. Mol Pharmacol. 2005 Dec;68(6):1590-6. | ||||
| 20 | PharmGKB summary: pathways of acetaminophen metabolism at the therapeutic versus toxic doses. Pharmacogenet Genomics. 2015 Aug;25(8):416-26. | ||||
| 21 | Induction of hepatic CYP2E1 by a subtoxic dose of acetaminophen in rats: increase in dichloromethane metabolism and carboxyhemoglobin elevation. Drug Metab Dispos. 2007 Oct;35(10):1754-8. | ||||
| 22 | Polymorphic expression of UGT1A9 is associated with variable acetaminophen glucuronidation in neonates: a population pharmacokinetic and pharmacogenetic study. Clin Pharmacokinet. 2018 Oct;57(10):1325-1336. | ||||
| 23 | Interindividual variability in acetaminophen sulfation by human fetal liver: implications for pharmacogenetic investigations of drug-induced birth defects. Birth Defects Res A Clin Mol Teratol. 2008 Mar;82(3):155-65. | ||||
| 24 | UGT1A6 and UGT2B15 polymorphisms and acetaminophen conjugation in response to a randomized, controlled diet of select fruits and vegetables. Drug Metab Dispos. 2011 Sep;39(9):1650-7. | ||||
| 25 | Characterization of niflumic acid as a selective inhibitor of human liver microsomal UDP-glucuronosyltransferase 1A9: application to the reaction phenotyping of acetaminophen glucuronidation. Drug Metab Dispos. 2011 Apr;39(4):644-52. | ||||
| 26 | The bacterial P450 BM3: a prototype for a biocatalyst with human P450 activities. Trends Biotechnol. 2007 Jul;25(7):289-98. | ||||
| 27 | Multiple microRNAs function as self-protective modules in acetaminophen-induced hepatotoxicity in humans. Arch Toxicol. 2018 Feb;92(2):845-858. | ||||
| 28 | Increased mitochondrial ROS formation by acetaminophen in human hepatic cells is associated with gene expression changes suggesting disruption of the mitochondrial electron transport chain. Toxicol Lett. 2015 Apr 16;234(2):139-50. | ||||
| 29 | Predictive toxicology using systemic biology and liver microfluidic "on chip" approaches: application to acetaminophen injury. Toxicol Appl Pharmacol. 2012 Mar 15;259(3):270-80. | ||||
| 30 | Gene expression analysis of precision-cut human liver slices indicates stable expression of ADME-Tox related genes. Toxicol Appl Pharmacol. 2011 May 15;253(1):57-69. | ||||
| 31 | Blood transcript immune signatures distinguish a subset of people with elevated serum ALT from others given acetaminophen. Clin Pharmacol Ther. 2016 Apr;99(4):432-41. | ||||
| 32 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 33 | Clark JM, Seager SJ "Gastric emptying following premedication with glycopyrrolate or atropine." Br J Anaesth 55 (1983): 1195-9. [PMID: 6652009] | ||||
| 34 | Product Information. Aubagio (teriflunomide). Genzyme Corporation, Cambridge, MA. | ||||
| 35 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 36 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 37 | Douidar SM, Ahmed AE "A novel mechanism for the enhancement of acetaminophen hepatotoxicity by phenobarbital." J Pharmacol Exp Ther 240 (1987): 578-83. [PMID: 3806412] | ||||
| 38 | Dybing E "Inhibition of acetaminophen glucuronidation by oxazepam." Biochem Pharmacol 25 (1976): 1421-5. [PMID: 938564] | ||||
| 39 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 40 | Bock KW, Wiltfang J, Blume R, Ullrich D, Bircher J "Paracetamol as a test drug to determine glucuronide formation in man: effects of inducers and of smoking." Eur J Clin Pharmacol 31 (1987): 677-83. [PMID: 3556375] | ||||
| 41 | Antlitz AM, Mead JA Jr, Tolentino MA "Potentiation of oral anticoagulant therapy by acetaminophen." Curr Ther Res Clin Exp 10 (1968): 501-7. [PMID: 4971464] | ||||
| 42 | Bonkovsky HL "Acetaminophen hepatotoxicity, fasting, and ethanol." JAMA 274 (1995): 301. [PMID: 7609254] | ||||
| 43 | Bivins BA, Rapp RP, Griffen WO Jr, Blouin R, Bustrack J "Dopamine-phenytoin interaction. A cause of hypotension in the critically ill." Arch Surg 113 (1978): 245-9. [PMID: 637689] | ||||
| 44 | Miners JO, Attwood J, Birkett DJ "Determinants of acetaminophen metabolism: effect of inducers and inhibitors of drug metabolism on acetaminophen's metabolic pathways." Clin Pharmacol Ther 35 (1984): 480-6. [PMID: 6705446] | ||||
| 45 | Crippin JS "Acetaminophen hepatotoxicity: potentiation by isoniazid." Am J Gastroenterol 88 (1993): 590-2. [PMID: 8470644] | ||||
| 46 | Product Information. Adcetris (brentuximab vedotin). Seattle Genetics Inc, Bothell, WA. | ||||
| 47 | Ameer B "Acetaminophen hepatotoxicity augmented by zidovudine." Am J Med 95 (1993): 342. [PMID: 8368234] | ||||
| 48 | Elsharkawy AM, Schwab U, McCarron B, et al. "Efavirenz induced acute liver failure requiring liver transplantation in a slow drug metaboliser." J Clin Virol 58 (2013): 331-3. [PMID: 23763943] | ||||
| 49 | Product Information. Kynamro (mipomersen). Genzyme Corporation, Cambridge, MA. | ||||
| 50 | Canadian Pharmacists Association. | ||||
| 51 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 52 | Product Information. Givlaari (givosiran). Alnylam Pharmaceuticals, Cambridge, MA. | ||||
| 53 | Al-Nawakil C, Willems L, Mauprivez C, et.al "Successful treatment of l-asparaginase-induced severe acute hepatotoxicity using mitochondrial cofactors." Leuk Lymphoma 55 (2014): 1670-4. [PMID: 24090500] | ||||
| 54 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 55 | Product Information. Clolar (clofarabine). sanofi-aventis, Bridgewater, NJ. | ||||
| 56 | Product Information. Zelboraf (vemurafenib). Genentech, South San Francisco, CA. | ||||
| 57 | Baraka OZ, Truman CA, Ford JM, Roberts CJ. The effect of propranolol on paracetamol metabolism in man.?Br J Clin Pharmacol. 1990;29(2):261-264. [PMID: 2306420] | ||||
| 58 | Product Information. Exjade (deferasirox). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 59 | Lin NU, Sarantopoulos S, Stone JR, et al. "Fatal hepatic necrosis following imatinib mesylate therapy." Blood 102 (2003): 3455-6. [PMID: 14568907] | ||||
| 60 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 61 | Jack D, Thomas M, Skidmore IF "Ranitidine and paracetamol metabolism." Lancet 2 (1985): 1067. [PMID: 2865542] | ||||
| 62 | Product Information. ReVia (naltrexone). DuPont Pharmaceuticals, Wilmington, DE. | ||||
| 63 | Product Information. Victoza (liraglutide). Novo Nordisk Pharmaceuticals Inc, Princeton, NJ. | ||||
