Details of the Drug
General Information of Drug (ID: DMGEMB7)
| Drug Name |
Hydrocortisone
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Acticort; Alacort; Algicirtis; Alphaderm; Amberin; Anflam; Anucort; Aquacort; Cetacort; Chronocort; Cleiton; Cobadex; Colocort; Corhydron; Cortanal; Cortef; Cortenema; Cortesal; Corticreme; Cortifair; Cortifan; Cortiment; Cortisol; Cortisolonum; Cortisporin; Cortispray; Cortolotion; Cortonema; Cortoxide; Cortril; Cremesone; Cutisol; Delacort; Dermacort; Dermaspray; Dermil; Dermocortal; Dermolate; Dihydrocostisone; Dioderm; Drotic; DuoCort; Efcorbin; Efcortelan; Efcortelin; Eldecort; Eldercort; Epicort; Epicortisol; Evacort; Ficortril; Fiocortril; Flexicort; Genacort; Glycort; Hidalone; Hycort; Hycortol; Hycortole; Hydracort; Hydrasson; Hydrocortal; Hydrocorticosterone; Hydrocortisyl; Hydrocortone; Hydroskin; Hydroxycortisone; Hytisone; Hytone; Idrocortisone; Kyypakkaus; Lactisona; Lubricort; Maintasone; Medicort; Meusicort; Mildison; Milliderm; Nutracort; Optef; Otocort; Penecort; Permicort; Prepcort; Proctocort; Protocort; Rectoid; Sanatison; Schericur; Sigmacort; Signef; Stiefcorcil; Synacort; Tarcortin; Texacort; Timocort; Traumaide; Uniderm; Vytone; Zenoxone; ACETASOL HC; Aeroseb HC; AnusolHC; Aquanil HC; Barseb HC; CaldeCORT Spray; Clear aid; Component of Lubricort; Component of Otalgine; Cortisol alcohol; Cortisporin Otico; Ef corlin; Epiderm H; Esiderm H; Foille Insetti; HYDROCORTISONE AND ACETIC ACID; HYDROCORTISONE IN ABSORBASE; Heb Cort; Hydrocortisone alcohol; Hydrocortisone base; Hydrocortisone free alcohol; Hydrocortisone solution; Hytone lotion; Idrocortisone [DCIT]; Komed HC; Lacticare HC; Nogenic HC; ORLEX HC; Pediotic Suspension; Polcort H; Preparation H Hydrocortisone Cream; Prevex HC; Proctozone HC; Remederm HC; Scalpicin Capilar; Scheroson F; Systral Hydrocort; Transderma H; VoSol HC; H 4001; Texacort lotion 25; [3H]cortisol; ACETIC ACID W/ HYDROCORTISONE; Acticort (TN); Aeroseb-HC; Ala-Cort; Ala-Scalp; Anti-inflammatory hormone; Anucort-HC; Anusol HC (TN); Balneol-hc; Basan-Corti; Beta-hc; COR-OTICIN; Colocort (TN); Cort-Dome; Cortef (TN); Cortisol, Hydrocortisone; Cremicort-H; Cyclodextrin-encapsulated hydrocortisone; Derm-Aid; Dome-cort; Domolene-HC; Genacort (lotion); Gyno-Cortisone; H-Cort; HC #1; HC #4; HC (HYDROCORTISONE); Heb-Cort; Hi-cor; Hidro-Colisona; Hidrocortisona [INN-Spanish]; Hydro-Adreson; Hydro-Colisona; Hydro-RX; Hydrocortisone-Water Soluble; Hydrocortisonum [INN-Latin]; Hytone (TN); Incortin-H; Incortin-hydrogen; Kendall's compound F; Lacticare-HC; Neo-Cortef; Neosporin-H Ear; Nystaform-HC; Otosone-F; Rectasol-HC; Reichstein's substance M; Scalp-Cort; Stie-cort; Component of Neo-Cort-Dome; Hydrocortisone [INN:BAN:JAN]; Hydrocortisone (JP15/USP/INN); 11-beta-Hydrocortisone; 11-beta-Hydroxycortisone; 11beta,17,21-Trihydroxyprogesterone; 11beta-Hydrocortisone; 11beta-Hydroxycortisone; 17-Hydroxycorticosterone; 17alpha-Hydroxycorticosterone
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiinflammatory Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
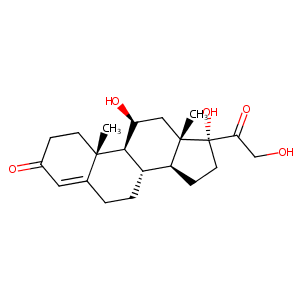 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 362.5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Hydrocortisone
Coadministration of a Drug Treating the Disease Different from Hydrocortisone (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Hydrocortisone FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2868). | ||||
| 3 | Hydrocortisone for COVID-19 and Severe Hypoxia (COVID STEROID) | ||||
| 4 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | ||||
| 5 | BDDCS applied to over 900 drugs | ||||
| 6 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 7 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 8 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | ||||
| 9 | Oral and inhaled corticosteroids: differences in P-glycoprotein (ABCB1) mediated efflux. Toxicol Appl Pharmacol. 2012 May 1;260(3):294-302. | ||||
| 10 | Regulation of the CYP3A4 gene by hydrocortisone and xenobiotics: role of the glucocorticoid and pregnane X receptors. Drug Metab Dispos. 2000 May;28(5):493-6. | ||||
| 11 | CYP3A5 genotype is associated with elevated blood pressure. Pharmacogenet Genomics. 2005 Oct;15(10):737-41. | ||||
| 12 | Steroid hydroxylation by human fetal CYP3A7 and human NADPH-cytochrome P450 reductase coexpressed in insect cells using baculovirus. Res Commun Mol Pathol Pharmacol. 1998 Apr;100(1):15-28. | ||||
| 13 | Studies on the origin of circulating 18-hydroxycortisol and 18-oxocortisol in normal human subjects. J Clin Endocrinol Metab. 2004 Sep;89(9):4628-33. | ||||
| 14 | Testosterone stimulates adipose tissue 11beta-hydroxysteroid dehydrogenase type 1 expression in a depot-specific manner in children. J Clin Endocrinol Metab. 2010 Jul;95(7):3300-8. | ||||
| 15 | Glucocorticoid-activation system mediated glucocorticoid-insulin-like growth factor 1 (GC-IGF1) axis programming alteration of adrenal dysfunction induced by prenatal caffeine exposure. Toxicol Lett. 2019 Mar 1;302:7-17. | ||||
| 16 | Towards a systematic analysis of human short-chain dehydrogenases/reductases (SDR): Ligand identification and structure-activity relationships. Chem Biol Interact. 2015 Jun 5;234:114-25. | ||||
| 17 | Glucocorticoid programming mechanism for hypercholesterolemia in prenatal ethanol-exposed adult offspring rats. Toxicol Appl Pharmacol. 2019 Jul 15;375:46-56. | ||||
| 18 | Prenatal caffeine exposure increases the susceptibility to non-alcoholic fatty liver disease in female offspring rats via activation of GR-C/EBP-SIRT1 pathway. Toxicology. 2019 Apr 1;417:23-34. doi: 10.1016/j.tox.2019.02.008. Epub 2019 Feb 15. | ||||
| 19 | Ultradian cortisol pulsatility encodes a distinct, biologically important signal. PLoS One. 2011 Jan 18;6(1):e15766. | ||||
| 20 | Human and murine steroid 5-reductases (AKR1D1 and AKR1D4): insights into the role of the catalytic glutamic acid. Chem Biol Interact. 2019 May 25;305:163-170. doi: 10.1016/j.cbi.2019.03.025. Epub 2019 Mar 28. | ||||
| 21 | P2Y receptor regulation of sodium transport in human mammary epithelial cells. Am J Physiol Cell Physiol. 2007 Nov;293(5):C1472-80. doi: 10.1152/ajpcell.00068.2007. Epub 2007 Aug 22. | ||||
| 22 | Baer PA, Shore A, Ikeman RL "Transient fall in serum salicylate levels following intraarticular injection of steroid in patients with rheumatoid arthritis." Arthritis Rheum 30 (1987): 345-7. [PMID: 3566826] | ||||
| 23 | Buchman AL, Schwartz MR "Colonic ulceration associated with the systemic use of nonsteroidal antiinflammatory medication." J Clin Gastroenterol 22 (1996): 224-6. [PMID: 8724264] | ||||
| 24 | Albin H, Vincon G, Demotes-Mainard F, et al "Effect of aluminium phosphate on the bioavailability of cimetidine and prednisolone." Eur J Clin Pharmacol 26 (1984): 271-3. [PMID: 6723769] | ||||
| 25 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 26 | Product Information. Multaq (dronedarone). sanofi-aventis , Bridgewater, NJ. | ||||
| 27 | Brooks SM, Werk EE, Ackerman SJ, Sullivan I, Thrasher K "Adverse effects of phenobarbital on corticosteroid metabolism in patients with bronchial asthma." N Engl J Med 286 (1972): 1125-8. [PMID: 4553339] | ||||
| 28 | Agencia Espaola de Medicamentos y Productos Sanitarios Healthcare "Centro de informacion online de medicamentos de la AEMPS - CIMA.". | ||||
| 29 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 30 | FDA. U.S. Food and Drug Administration "Information for Healthcare Professionals. Fluoroquinolone Antimicrobial Drugs. FDA Alert [7/8/2008].". | ||||
| 31 | Product Information. Synercid (dalfopristin-quinupristin) Rhone-Poulenc Rorer, Collegeville, PA. | ||||
| 32 | Product Information. Tykerb (lapatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 33 | Product Information. Korlym (mifepristone). Corcept Therapeutics Incorporated, Menlo Park, CA. | ||||
| 34 | Product Information. Kalydeco (ivacaftor). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 35 | Product Information. Prevymis (letermovir). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 36 | Bartoszek M, Brenner AM, Szefler SJ "Prednisolone and methylprednisolone kinetics in children receiving anticonvulsant therapy." Clin Pharmacol Ther 42 (1987): 424-32. [PMID: 3665340] | ||||
| 37 | Product Information. Trileptal (oxcarbazepine) Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 38 | Product Information. Xcopri (cenobamate). SK Life Science, Inc., Paramus, NJ. | ||||
| 39 | EMEA. European Medicines Agency "EPARs. European Union Public Assessment Reports.". | ||||
| 40 | Frey BM, Frey FJ "Phenytoin modulates the pharmacokinetics of prednisolone and the pharmacodynamics of prednisolone as assessed by the inhibition of the mixed lymphocyte reaction in humans." Eur J Clin Invest 14 (1984): 1-6. [PMID: 6230241] | ||||
| 41 | Product Information. Tazverik (tazemetostat). Epizyme, Inc, Cambridge, MA. | ||||
| 42 | Edsbacker S, Andersson T "Pharmacokinetics of budesonide (Entocort EC) capsules for Crohn's disease." Clin Pharmacokinet 43 (2004): 803-21. [PMID: 15355126] | ||||
| 43 | Product Information. Incivek (telaprevir). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 44 | Kyriazopoulou V, Parparousi O, Vagenakis AG "Rifampicin-induced adrenal crisis in Addisonian patients receiving corticosteroid replacement therapy." J Clin Endocrinol Metab 59 (1984): 1204-6. [PMID: 6490796] | ||||
| 45 | Product Information. Priftin (rifapentine). Hoechst Marion-Roussel Inc, Kansas City, MO. | ||||
| 46 | Product Information. Intelence (etravirine). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 47 | Product Information. Nexletol (bempedoic acid). Esperion Therapeutics, Ann Arbor, MI. | ||||
| 48 | Product Information. Arava (leflunomide). Hoechst Marion-Roussel Inc, Kansas City, MO. | ||||
| 49 | Messer J, Reitman D, Sacks HS, et al "Association of adrenocorticosteroid therapy and peptic-ulcer disease." N Engl J Med 309 (1983): 21-4. [PMID: 6343871] | ||||
| 50 | Product Information. Prolia (denosumab). Amgen USA, Thousand Oaks, CA. | ||||
| 51 | Product Information. Copiktra (duvelisib). Verastem, Inc., Needham, MA. | ||||
| 52 | Ohnishi K, Yoshida H, Shigeno K, et al. "Prolongation of the QT interval and ventricular tachycardia in patients treated with arsenic trioxide for acute promyelocytic leukemia." Ann Intern Med 133 (2000): 881-5. [PMID: 11103058] | ||||
| 53 | Bennett CL, Nebeker JR, Samore MH, et al "The Research on Adverse Drug Events and Reports (RADAR) project." JAMA 293 (2005): 2131-40. [PMID: 15870417] | ||||
| 54 | Product Information. Vumerity (diroximel fumarate). Alkermes, Inc, Cambridge, MA. | ||||
| 55 | Product Information. Gilenya (fingolimod). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 56 | Product Information. Ocrevus (ocrelizumab). Genentech, South San Francisco, CA. | ||||
| 57 | Product Information. Tasigna (nilotinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 58 | Product Information. Sprycel (dasatinib). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 59 | Product Information. Synribo (omacetaxine). Teva Pharmaceuticals USA, North Wales, PA. | ||||
| 60 | Doherty MM, Charman WN "The mucosa of the small intestine: how clinically relevant as an organ of drug metabolism?" Clin Pharmacokinet 41 (2002): 235-53. [PMID: 11978143] | ||||
| 61 | Product Information. Wellbutrin XL (buPROPion). GlaxoSmithKline, Philadelphia, PA. | ||||
| 62 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 63 | Hansen RA, Tu W, Wang J, Ambuehl R, McDonald CJ, Murray MD "Risk of adverse gastrointestinal events from inhaled corticosteroids." Pharmacotherapy 28 (2008): 1325-34. [PMID: 18956992] | ||||
| 64 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 65 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 66 | Benoist G, van Oort I, et al "Drug-drug interaction potential in men treated with enzalutamide: Mind the gap." Br J Clin Pharmacol 0 (2017): epub. [PMID: 28881501] | ||||
| 67 | Product Information. Prograf (tacrolimus). Fujisawa, Deerfield, IL. | ||||
| 68 | Product Information. Arcalyst (rilonacept). Regeneron Pharmaceuticals Inc, Tarrytown, NY. | ||||
| 69 | Product Information. Cimzia (certolizumab). UCB Pharma Inc, Smyrna, GA. | ||||
| 70 | Product Information. Orap Tablets (pimozide). Gate Pharmaceuticals, Sellersville, PA. | ||||
| 71 | CDC. Centers for Disease Control and Prevention/ "Recommendations of the advisory committtee on immunization practices (ACIP): use of vaccines and immune globulins in persons with altered immunocompetence." MMWR Morb Mortal Wkly Rep 42(RR-04) (1993): 1-18. [PMID: 20300058] | ||||
| 72 | DSouza DL, Levasseur LM, Nezamis J, Robbins DK, Simms L, Koch KM "Effect of alosetron on the pharmacokinetics of alprazolam." J Clin Pharmacol 41 (2001): 452-4. [PMID: 11304902] | ||||
| 73 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
| 74 | Antonelli D, Atar S, Freedberg NA, Rosenfeld T "Torsade de pointes in patients on chronic amiodarone treatment: contributing factors and drug interactions." Isr Med Assoc J 7 (2005): 163-5. [PMID: 15792261] | ||||
