Details of the Drug
General Information of Drug (ID: DMNH3PG)
| Drug Name |
Etoposide
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
etoposide; 33419-42-0; VePesid; Toposar; trans-Etoposide; Lastet; (-)-Etoposide; Zuyeyidal; Etoposidum; Etoposido; Vepesid J; Eposin; Etoposidum [INN-Latin]; Etoposide (VP16); VP 16-213; VP 16 (pharmaceutical); Etoposido [INN-Spanish]; Etopophos (phosphate salt); VP-16-213; 4-Demethylepipodophyllotoxin beta-D-ethylideneglucoside; VP 16213; UNII-6PLQ3CP4P3; NK 171; NSC 141540; CCRIS 2392; HSDB 6517; 4'-Demethylepipodophyllotoxin 9-(4,6-O-(R)-ethylidene-beta-D-glucopyranoside); EINECS 251-509-1; NSC-141540; Eposide; Etopol; Etosid; Vepeside; Demethyl EpipodophyllotoxinEthylidine Glucoside; E0675; Demethyl-epiodophyllotoxin ethylidene glucoside; Epipodophyllotoxin VP-16213; Eposin (TN); Etopophos (TN); Trans-Etoposide; VePESID (TN); Vepesid (TN); DEMETHY-EPIPODOPHYLLOTOXIN, ETHYLIDENE GLUCOSIDE; VP-16 (TN); Demethylepipodophyllotoxin-beta-D-ethylideneglucoside; Etoposide (JP15/USP/INN); Etoposide [USAN:INN:BAN:JAN]; Eposin, Vepesid, VP-16, Toposar, Etoposide; Epipodophyllotoxin, 4'-demethyl-, 4,6-O-ethylidene-beta-D-glucopyranoside; Epipodophyllotoxin, 4'-demethyl-, 4,6-O-ethylidene-beta-D-glucopyranoside (8CI); Epipodophyllotoxin, 4'-demethyl-, 9-(4,6-O-ethylidene-beta-D-glucopyranoside); 4'-Demethyl-epipodophyllotoxin 9-[4,6-O-(R)-ethylidene-beta-D-glucopyranoside; 4'-Demethylepipodophyllotoxin 9-(4,6-O-ethylidene-beta-D-glucopyranoside); 4'-Demethylepipodophyllotoxin ethylidene-beta-D-glucoside; 4'-O-Demethyl-1-O-(4,6-O-ethylidene-beta-D-glucopyranosyl)epipodophyllotoxin; 4-Demethylepipodophyllotoxin-beta-D-ethylideneglucoside
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
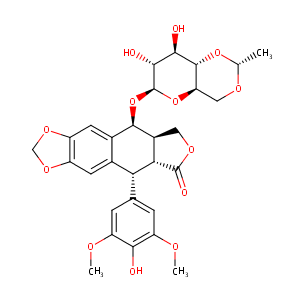 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 588.6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 13 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Experimental Cancer Drug Sensitivity Information
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Etoposide
Coadministration of a Drug Treating the Disease Different from Etoposide (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
| DIG |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pharmaceutical Formulation |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
| 1 | Etoposide FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6815). | ||||
| 3 | FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (ANDA) 075635. | ||||
| 4 | ClinicalTrials.gov (NCT04356690) Etoposide in Patients With COVID-19 Infection. U.S. National Institutes of Health. | ||||
| 5 | BDDCS applied to over 900 drugs | ||||
| 6 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 7 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 8 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 9 | Genome-wide association study of chemotherapeutic agent-induced severe neutropenia/leucopenia for patients in Biobank Japan. Cancer Sci. 2013 Aug;104(8):1074-82. doi: 10.1111/cas.12186. Epub 2013 Jun 10. | ||||
| 10 | Etoposide, topoisomerase II and cancer.Curr Med Chem Anticancer Agents.2005 Jul;5(4):363-72. | ||||
| 11 | Functional reconstitution of human ABCC3 into proteoliposomes reveals a transport mechanism with positive cooperativity. Biochemistry. 2009 May 26;48(20):4423-30. | ||||
| 12 | Delineating the contribution of secretory transporters in the efflux of etoposide using Madin-Darby canine kidney (MDCK) cells overexpressing P-glycoprotein (Pgp), multidrug resistance-associated protein (MRP1), and canalicular multispecific organic anion transporter (cMOAT). Drug Metab Dispos. 2002 Apr;30(4):457-63. | ||||
| 13 | Identification of drugs and drug metabolites as substrates of multidrug resistance protein 2 (MRP2) using triple-transfected MDCK-OATP1B1-UGT1A1-MRP2 cells. Br J Pharmacol. 2012 Mar;165(6):1836-1847. | ||||
| 14 | Multidrug resistance-associated protein-1 functional activity in Calu-3 cells. J Pharmacol Exp Ther. 2001 Sep;298(3):1199-205. | ||||
| 15 | Characterization of 3-methoxy flavones for their interaction with ABCG2 as suggested by ATPase activity. Biochim Biophys Acta. 2014 Nov;1838(11):2929-38. | ||||
| 16 | Effect of pregnane X receptor ligands on transport mediated by human OATP1B1 and OATP1B3. Eur J Pharmacol. 2008 Apr 14;584(1):57-65. | ||||
| 17 | Efflux ratio cannot assess P-glycoprotein-mediated attenuation of absorptive transport: asymmetric effect of P-glycoprotein on absorptive and secretory transport across Caco-2 cell monolayers. Pharm Res. 2003 Aug;20(8):1200-9. | ||||
| 18 | Effects of morin on the pharmacokinetics of etoposide in rats. Biopharm Drug Dispos. 2007 Apr;28(3):151-6. | ||||
| 19 | A study on the metabolism of etoposide and possible interactions with antitumor or supporting agents by human liver microsomes. J Pharmacol Exp Ther. 1998 Sep;286(3):1294-300. | ||||
| 20 | UDP-glucuronosyltransferase 1A1 is the principal enzyme responsible for etoposide glucuronidation in human liver and intestinal microsomes: structural characterization of phenolic and alcoholic glucuronides of etoposide and estimation of enzyme kinetics. Drug Metab Dispos. 2007 Mar;35(3):371-80. | ||||
| 21 | Kinetics and regulation of cytochrome P450-mediated etoposide metabolism. Drug Metab Dispos. 2004 Sep;32(9):993-1000. | ||||
| 22 | Peroxidative free radical formation and O-demethylation of etoposide(VP-16) and teniposide(VM-26). Biochem Biophys Res Commun. 1986 Feb 26;135(1):215-20. | ||||
| 23 | Reactions of glutathione with the catechol, the ortho-quinone and the semi-quinone free radical of etoposide. Consequences for DNA inactivation. Biochem Pharmacol. 1992 Apr 15;43(8):1761-8. | ||||
| 24 | Genomic profiling uncovers a molecular pattern for toxicological characterization of mutagens and promutagens in vitro. Toxicol Sci. 2011 Jul;122(1):185-97. | ||||
| 25 | Differential regulation of vitamin D receptor (VDR) by the p53 Family: p73-dependent induction of VDR upon DNA damage. J Biol Chem. 2007 Oct 12;282(41):29847-54. | ||||
| 26 | Gene expression profiling of 30 cancer cell lines predicts resistance towards 11 anticancer drugs at clinically achieved concentrations. Int J Cancer. 2006 Apr 1;118(7):1699-712. doi: 10.1002/ijc.21570. | ||||
| 27 | Utilization of CDKN1A/p21 gene for class discrimination of DNA damage-induced clastogenicity. Toxicology. 2014 Jan 6;315:8-16. doi: 10.1016/j.tox.2013.10.009. Epub 2013 Nov 6. | ||||
| 28 | Cell death mechanisms of the anti-cancer drug etoposide on human cardiomyocytes isolated from pluripotent stem cells. Arch Toxicol. 2018 Apr;92(4):1507-1524. | ||||
| 29 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 30 | Doherty MM, Charman WN "The mucosa of the small intestine: how clinically relevant as an organ of drug metabolism?" Clin Pharmacokinet 41 (2002): 235-53. [PMID: 11978143] | ||||
| 31 | DSouza DL, Levasseur LM, Nezamis J, Robbins DK, Simms L, Koch KM "Effect of alosetron on the pharmacokinetics of alprazolam." J Clin Pharmacol 41 (2001): 452-4. [PMID: 11304902] | ||||
| 32 | Cummings J, Forrest GJ, Cunningham D, Gilchrist NL, Soukop M "Influence of polysorbate 80 (tween 80) and etoposide (VP-16-213 on the pharmacokinetics and urinary excretion of adriamycin and its metabolites in cancer patients." Cancer Chemother Pharmacol 17 (1987): 80-4. [PMID: 3698181] | ||||
| 33 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 34 | Product Information. Multaq (dronedarone). sanofi-aventis , Bridgewater, NJ. | ||||
| 35 | Johnson EJ, MacGowan AP, Potter MN, et al "Reduced absorption of oral ciprofloxacin after chemotherapy for haematological malignancy." J Antimicrob Chemother 25 (1990): 837-42. [PMID: 2373666] | ||||
| 36 | Product Information. Synercid (dalfopristin-quinupristin) Rhone-Poulenc Rorer, Collegeville, PA. | ||||
| 37 | Product Information. Ketek (telithromycin). Aventis Pharmaceuticals, Bridgewater, NJ. | ||||
| 38 | Product Information. Balversa (erdafitinib). Janssen Products, LP, Horsham, PA. | ||||
| 39 | Product Information. Tykerb (lapatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 40 | Product Information. Tukysa (tucatinib). Seattle Genetics Inc, Bothell, WA. | ||||
| 41 | Le AT, Hasson NK, Lum BL "Enhancement of warfarin response in a patient receiving etoposide and carboplatin chemotherapy." Ann Pharmacother 31 (1997): 1006-8. [PMID: 9296241] | ||||
| 42 | Product Information. Kalydeco (ivacaftor). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 43 | Product Information. Prevymis (letermovir). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 44 | Product Information. Serzone (nefazodone). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 45 | Rodman JH, Murry DJ, Madden T, Santana VM "Altered etoposide pharmacokinetics and time to engraftment in pediatric patients undergoing autologous bone marrow transplantation." J Clin Oncol 12 (1994): 2390-7. [PMID: 7964955] | ||||
| 46 | Product Information. Xcopri (cenobamate). SK Life Science, Inc., Paramus, NJ. | ||||
| 47 | EMEA. European Medicines Agency "EPARs. European Union Public Assessment Reports.". | ||||
| 48 | Product Information. Tazverik (tazemetostat). Epizyme, Inc, Cambridge, MA. | ||||
| 49 | Auclair B, Berning SE, Huitt GA, Peloquin CP "Potential interaction between itraconazole and clarithromycin." Pharmacotherapy 19 (1999): 1439-44. [PMID: 10600094] | ||||
| 50 | Product Information. Victrelis (boceprevir). Schering-Plough Corporation, Kenilworth, NJ. | ||||
| 51 | Product Information. Incivek (telaprevir). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 52 | Product Information. Harvoni (ledipasvir-sofosbuvir). Gilead Sciences, Foster City, CA. | ||||
| 53 | Product Information. Priftin (rifapentine). Hoechst Marion-Roussel Inc, Kansas City, MO. | ||||
| 54 | Carrion C, Espinosa E, Herrero A, Garcia B "Possible vincristine-isoniazid interaction." Ann Pharmacother 29 (1995): 201. [PMID: 7756727] | ||||
| 55 | Product Information. Fortovase (saquinavir) Roche Laboratories, Nutley, NJ. | ||||
| 56 | Product Information. Intelence (etravirine). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 57 | Argov Z, Mastaglia FL "Drug-induced peripheral neuropathies." Br Med J 1 (1979): 663-6. [PMID: 219931] | ||||
| 58 | Product Information. Prezista (darunavir). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 59 | Product Information. Arava (leflunomide). Hoechst Marion-Roussel Inc, Kansas City, MO. | ||||
| 60 | Product Information. Vaprisol (conivaptan). Cumberland Pharmaceuticals Inc, Nashville, TN. | ||||
| 61 | Product Information. Prolia (denosumab). Amgen USA, Thousand Oaks, CA. | ||||
| 62 | Product Information. Alunbrig (brigatinib). Ariad Pharmaceuticals Inc, Cambridge, MA. | ||||
| 63 | Product Information. Lorbrena (lorlatinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 64 | Product Information. Tabrecta (capmatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 65 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 66 | Product Information. Copiktra (duvelisib). Verastem, Inc., Needham, MA. | ||||
| 67 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 68 | Product Information. Exjade (deferasirox). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 69 | Bennett CL, Nebeker JR, Samore MH, et al "The Research on Adverse Drug Events and Reports (RADAR) project." JAMA 293 (2005): 2131-40. [PMID: 15870417] | ||||
| 70 | Product Information. Vumerity (diroximel fumarate). Alkermes, Inc, Cambridge, MA. | ||||
| 71 | Product Information. Gilenya (fingolimod). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 72 | Product Information. Ocrevus (ocrelizumab). Genentech, South San Francisco, CA. | ||||
| 73 | Product Information. Tasigna (nilotinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 74 | Product Information. Sprycel (dasatinib). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 75 | Product Information. Synribo (omacetaxine). Teva Pharmaceuticals USA, North Wales, PA. | ||||
| 76 | Product Information. Varubi (rolapitant). Tesaro Inc., Waltham, MA. | ||||
| 77 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 78 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 79 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 80 | Product Information. Zokinvy (lonafarnib). Eiger BioPharmaceuticals, Palo Alto, CA. | ||||
| 81 | Benoist G, van Oort I, et al "Drug-drug interaction potential in men treated with enzalutamide: Mind the gap." Br J Clin Pharmacol 0 (2017): epub. [PMID: 28881501] | ||||
| 82 | Product Information. Prograf (tacrolimus). Fujisawa, Deerfield, IL. | ||||
| 83 | Product Information. Arcalyst (rilonacept). Regeneron Pharmaceuticals Inc, Tarrytown, NY. | ||||
| 84 | Product Information. Cimzia (certolizumab). UCB Pharma Inc, Smyrna, GA. | ||||
| 85 | CDC. Centers for Disease Control and Prevention/ "Recommendations of the advisory committtee on immunization practices (ACIP): use of vaccines and immune globulins in persons with altered immunocompetence." MMWR Morb Mortal Wkly Rep 42(RR-04) (1993): 1-18. [PMID: 20300058] | ||||
| 86 | Product Information. Oxbryta (voxelotor). Global Blood Therapeutics, Inc., South San Francisco, CA. | ||||
| 87 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
