Details of the Drug
General Information of Drug (ID: DMTK234)
| Drug Name |
Piroxicam
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Akten; Artroxicam; Bruxicam; Caliment; Erazon; Felden; Feldene; Flogobene; Geldene; Improntal; Larapam; Pipoxicam; Pirkam; Piroflex; Piroftal; Piroxicamum; Pyroxycam; Reudene; Riacen; Roxicam; Roxiden; Sasulen; Solocalm; Zunden; Feldene Fast; Feldene Gel; Piroxicam usp; AK1015; CHF 1251; CP 16171; CP16171; P 5654; Apo-Piroxicam; Brexidol (TN); Brexin (TN); CP-16171; Erazon (TN); Exipan (TN); Felden (TN); Feldene (TN); Feldoral (TN); Hotemin (TN); Mobilis (TN); Pirox von ct (TN); Piroxicamum [INN-Latin]; Proponol (TN); Reumador (TN); Tracam (TN); Veral (TN); Vurdon (TN); Feldene, Roxam, Piroxicam; Piroxicam (JP15/USP/INN); Piroxicam [USAN:BAN:INN:JAN]; (3E)-3-[hydroxy-(pyridin-2-ylamino)methylidene]-2-methyl-1,1-dioxo-1; (4-Hydroxy-2-methyl-1,1-dioxobenzo[e]1,2-thiazin-3-yl)-N-(2-pyridyl)carboxamide; 2H-1,2-Benzothiazine-3-carboxamide, 4-hydroxy-2-methyl-N-2-pyridinyl-, 1,1-dioxide; 3-[hydroxy(pyridin-2-ylamino)methylidene]-2-methyl-3,4-dihydro-2H-1; 4-Hydroxy-2-methyl-3-(pyrid-2-yl-carbamoyl)-2H-1,2-benzothiazine 1,1-dioxide; 4-Hydroxy-2-methyl-N-(2-pyridyl)-2H-1,2-benzothiazin-3-caboxyamid-1,1-dioxid; 4-Hydroxy-2-methyl-N-(2-pyridyl)-2H-1,2-benzothiazin-3-caboxyamid-1,1-dioxid [German]; 4-Hydroxy-2-methyl-N-(2-pyridyl)-2H-1,2-benzothiazine-3-carboxamide-1,1-dioxide; 4-Hydroxy-2-methyl-N-2-pyridyl-2H-1,2-benzothiazine-3-carboxamide 1,1-dioxide; 4-hydroxy-2-methyl-N-(pyridin-2-yl)-2H-1,2-benzothiazine-3-carboxamide 1,1-dioxide; 4-hydroxy-2-methyl-N-pyridin-2-yl-2H-1,2-benzothiazine-3-carboxamide 1,1-dioxide
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Analgesics
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
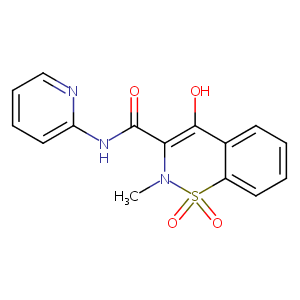 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 331.3 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.1 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Osteoarthritis | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | FA00-FA05 | |||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Piroxicam
Coadministration of a Drug Treating the Disease Different from Piroxicam (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Piroxicam FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7273). | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 5 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 8 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | ||||
| 9 | Functional characterization of human CYP2C9 allelic variants in COS-7 cells. Front Pharmacol. 2016 Apr 25;7:98. | ||||
| 10 | Efficacy of piroxicam for postoperative pain after lower third molar surgery associated with CYP2C8*3 and CYP2C9 J Pain Res. 2017 Jul 6;10:1581-1589. | ||||
| 11 | Apoptosis induced by piroxicam plus cisplatin combined treatment is triggered by p21 in mesothelioma. PLoS One. 2011;6(8):e23569. | ||||
| 12 | Antirheumatic drug response signatures in human chondrocytes: potential molecular targets to stimulate cartilage regeneration. Arthritis Res Ther. 2009;11(1):R15. | ||||
| 13 | Abdel-Rahman MS, Reddi AS, Curro FA, Turkall RM, Kadry AM, Hansrote JA "Bioavailability of aspirin and salicylamide following oral co-administration in human volunteers." Can J Physiol Pharmacol 69 (1991): 1436-42. [PMID: 1777842] | ||||
| 14 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 15 | Abad S, Moachon L, Blanche P, Bavoux F, Sicard D, Salmon-Ceron D "Possible interaction between glicazide, fluconazole and sulfamethoxazole resulting in severe hypoglycaemia." Br J Clin Pharmacol 52 (2001): 456-7. [PMID: 11678792] | ||||
| 16 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 17 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 18 | Product Information. Acular (ketorolac). Allergan Inc, Irvine, CA. | ||||
| 19 | Buchman AL, Schwartz MR "Colonic ulceration associated with the systemic use of nonsteroidal antiinflammatory medication." J Clin Gastroenterol 22 (1996): 224-6. [PMID: 8724264] | ||||
| 20 | Product Information. Factive (gemifloxacin). GeneSoft Inc, San Francisco, CA. | ||||
| 21 | Assael BM, Chiabrando C, Gagliardi L, Noseda A, Bamonte F, Salmona M "Prostaglandins and aminoglycoside nephrotoxicity." Toxicol Appl Pharmacol 78 (1985): 386-94. [PMID: 4049389] | ||||
| 22 | Product Information. Actonel (risedronate). Procter and Gamble Pharmaceuticals, Cincinnati, OH. | ||||
| 23 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 24 | Product Information. Piqray (alpelisib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 25 | Wong GT, Lee EY, Irwin MG. Contrast induced nephropathy in vascular surgery.?Br J Anaesth. 2016;117 Suppl 2:ii63-ii73. [PMID: 27566809] | ||||
| 26 | Product Information. Yasmin (drospirenone-ethinyl estradiol) Berlex Laboratories, Richmond, CA. | ||||
| 27 | Bang CJ, Riedel B, Talstad I, Berstad A "Interaction between heparin and acetylsalicylic acid on gastric mucosal and skin bleeding in humans." Scand J Gastroenterol 27 (1992): 489-94. [PMID: 1321488] | ||||
| 28 | Product Information. Korlym (mifepristone). Corcept Therapeutics Incorporated, Menlo Park, CA. | ||||
| 29 | Product Information. Kalydeco (ivacaftor). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 30 | Product Information. Prevymis (letermovir). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 31 | Product Information. Xarelto (rivaroxaban). Bayer Inc, Toronto, IA. | ||||
| 32 | Product Information. Emend (aprepitant). Merck & Company Inc, West Point, PA. | ||||
| 33 | Alderman CP, Moritz CK, Ben-Tovim DI "Abnormal platelet aggregation associated with fluoxetine therapy." Ann Pharmacother 26 (1992): 1517-9. [PMID: 1482806] | ||||
| 34 | Canadian Pharmacists Association. | ||||
| 35 | Muller FO, Schall R, Devaal AC, Groenewoud G, Hundt HKL, Middle MV "Influence of meloxicam on furosemide pharmacokinetics and pharmacodynamics in healthy volunteers." Eur J Clin Pharmacol 48 (1995): 247-51. [PMID: 7589049] | ||||
| 36 | Abdel-Haq B, Magagna A, Favilla S, Salvetti A "Hemodynamic and humoral interactions between perindopril and indomethacin in essential hypertensive subjects." J Cardiovasc Pharmacol 18 (1991): s33-6. [PMID: 1725198] | ||||
| 37 | Product Information. Priftin (rifapentine). Hoechst Marion-Roussel Inc, Kansas City, MO. | ||||
| 38 | Product Information. Adcetris (brentuximab vedotin). Seattle Genetics Inc, Bothell, WA. | ||||
| 39 | Elsharkawy AM, Schwab U, McCarron B, et al. "Efavirenz induced acute liver failure requiring liver transplantation in a slow drug metaboliser." J Clin Virol 58 (2013): 331-3. [PMID: 23763943] | ||||
| 40 | Product Information. Intelence (etravirine). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 41 | Product Information. Kynamro (mipomersen). Genzyme Corporation, Cambridge, MA. | ||||
| 42 | EMEA "EMEA public statement on leflunomide (ARAVA) - severe and serious hepatic reactions.". | ||||
| 43 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 44 | McCarthy JT, Torres VE, Romero JC, et al "Acute intrinsic renal failure induced by indomethacin." Mayo Clin Proc 57 (1982): 289-96. [PMID: 6952058] | ||||
| 45 | Product Information. Potassium Chloride ER (potassium chloride). Zydus Pharmaceuticals (USA) Inc, Princeton, NJ. | ||||
| 46 | Novis BH, Korzets Z, Chen P, Bernheim J "Nephrotic syndrome after treatment with 5-aminosalicylic acid." Br Med J (Clin Res Ed) 296 (1988): 1442. [PMID: 3132281] | ||||
| 47 | Adams JD, Hunter GA "Drug interaction in psoriasis." Australas J Dermatol 17 (1976): 39-40. [PMID: 1022213] | ||||
| 48 | Blakely KM, Drucker AM, Rosen CF "Drug-induced photosensitivity-an update: Culprit drugs, prevention and management." Drug Saf 42 (2019): 827-47. [PMID: 30888626] | ||||
| 49 | Caruso V, Iacoviello L, Di Castelnuovo A, et.al "Thrombotic complications in childhood acute lymphoblastic leukemia: a meta-analysis of 17 prospective studies comprising 1752 pediatric patients." Blood 108 (2006): 2216-22. [PMID: 16804111] | ||||
| 50 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 51 | Product Information. Calquence (acalabrutinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 52 | Agencia Espaola de Medicamentos y Productos Sanitarios Healthcare "Centro de informacion online de medicamentos de la AEMPS - CIMA.". | ||||
| 53 | Product Information. Iclusig (ponatinib). Ariad Pharmaceuticals Inc, Cambridge, MA. | ||||
| 54 | Product Information. Exjade (deferasirox). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 55 | Product Information. Sprycel (dasatinib). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 56 | Product Information. Brukinsa (zanubrutinib). BeiGene USA, Inc, San Mateo, CA. | ||||
| 57 | Product Information. Zontivity (vorapaxar). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 58 | Product Information. Integrilin (eptifibatide). Schering Laboratories, Kenilworth, NJ. | ||||
| 59 | Product Information. Solaraze (diclofenac topical). Doak Dermatologics Division, Fairfield, NJ. | ||||
| 60 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 61 | Benoist G, van Oort I, et al "Drug-drug interaction potential in men treated with enzalutamide: Mind the gap." Br J Clin Pharmacol 0 (2017): epub. [PMID: 28881501] | ||||
| 62 | Product Information. Tracleer (bosentan). Acetelion Pharmaceuticals US, Inc, South San Francisco, CA. | ||||
| 63 | Product Information. Flolan (epoprostenol). Glaxo Wellcome, Research Triangle Park, NC. | ||||
| 64 | Product Information. Prograf (tacrolimus). Fujisawa, Deerfield, IL. | ||||
| 65 | Product Information. ReVia (naltrexone). DuPont Pharmaceuticals, Wilmington, DE. | ||||
| 66 | Product Information. Cometriq (cabozantinib). Exelixis Inc, S San Francisco, CA. | ||||
| 67 | Product Information. Bevyxxa (betrixaban). Portola Pharmaceuticals, South San Francisco, CA. | ||||
