Details of the Drug
General Information of Drug (ID: DMBDAZV)
| Drug Name |
Amlodipine
|
||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Amlocard; Amlodipino; Amlodipinum; Amlodis; Amlopres; Amlor; Coroval; Intervask; Lipinox; AMLODIPINE BASE; Amlodipine Free Base; Amlodipino [Spanish]; Amlodipinum [Latin]; Racemic Amlodipine; Amlodipine (INN); Amlodipine [INN:BAN]; Dailyvasc (TN); Istin (TN); Norvasc (TN); Perivasc (TN); UK-4834011; R,S)-Amlodipine; 2-[(2-Aminoethoxy)methyl]-4-(2-chlorophenyl); 3,5-Pyridinedicarboxylic acid, 2-((2-aminoethoxy)methyl)-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-, 3-ethyl 5-methyl ester; 3,5-Pyridinedicarboxylic acid, 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-, 3-ethyl 5-methyl ester; 3-Ethyl-5-methyl (+-)-2-(2-aminoethoxymethyl)-4-(o-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate; 3-Ethyl-5-methyl (+/-)-2-[(2-aminoethoxy)methyl]-4-(o-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate; 3-Ethyl-5-methyl (-)-2-((2-aminoethoxy)methyl)-4-(2-chlorphenyl)-1,4-dihydro-6-methyl-3,5-pyridindicarboxylat; 3-Ethyl-5-methyl (-)-2-((2-aminoethoxymethyl)-4-(o-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate; 3-O-ethyl 5-O-methyl 2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate; 3-ethyl 5-methyl 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate; 3-ethyl 5-methyl 2-{[(2-aminoethyl)oxy]methyl}-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antihypertensive Agents
|
||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
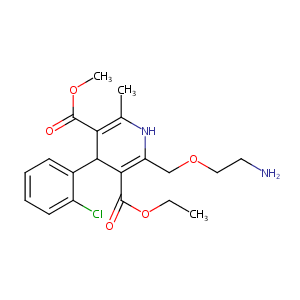 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 408.9 | |||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3 | ||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 10 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Angina pectoris | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | BA40 | |||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Amlodipine
Coadministration of a Drug Treating the Disease Different from Amlodipine (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Amlodipine FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6981). | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Meredith PA, Elliott HL: Clinical pharmacokinetics of amlodipine. Clin Pharmacokinet. 1992 Jan;22(1):22-31. doi: 10.2165/00003088-199222010-00003. | ||||
| 5 | Shore N, Zurth C, Fricke R, Gieschen H, Graudenz K, Koskinen M, Ploeger B, Moss J, Prien O, Borghesi G, Petrenciuc O, Tammela TL, Kuss I, Verholen F, Smith MR, Fizazi K: Evaluation of Clinically Relevant Drug-Drug Interactions and Population Pharmacokinetics of Darolutamide in Patients with Nonmetastatic Castration-Resistant Prostate Cancer: Results of Pre-Specified and Post Hoc Analyses of the Phase III ARAMIS Trial. Target Oncol. 2019 Oct;14(5):527-539. doi: 10.1007/s11523-019-00674-0. | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 8 | Pharmacogenetic association of the NPPA T2238C genetic variant with cardiovascular disease outcomes in patients with hypertension. JAMA. 2008 Jan 23;299(3):296-307. doi: 10.1001/jama.299.3.296. | ||||
| 9 | A first drug combination for the treatment of arterial hypertension with a calcium channel antagonist (amlodipine besylate) and an angiotensin receptor blocker (valsartan): Exforge. Rev Med Liege. 2007 Nov;62(11):688-94. | ||||
| 10 | Amlodipine metabolism in human liver microsomes and roles of CYP3A4/5 in the dihydropyridine dehydrogenation. Drug Metab Dispos. 2014 Feb;42(2):245-9. | ||||
| 11 | Amlodipine improves endothelial function and metabolic parameters in patients with hypertension. Int J Cardiol. 2009 Mar 20;133(1):23-31. doi: 10.1016/j.ijcard.2007.11.058. Epub 2008 Jan 15. | ||||
| 12 | Cell-based high-throughput screening for aromatase inhibitors in the Tox21 10K library. Toxicol Sci. 2015 Oct;147(2):446-57. | ||||
| 13 | G1 cell cycle arrest by amlodipine, a dihydropyridine Ca2+ channel blocker, in human epidermoid carcinoma A431 cells. Biochem Pharmacol. 2007 Apr 1;73(7):943-53. doi: 10.1016/j.bcp.2006.12.011. Epub 2006 Dec 14. | ||||
| 14 | Inhibition of human cytochrome P450 enzymes by 1,4-dihydropyridine calcium antagonists: prediction of in vivo drug-drug interactions. Eur J Clin Pharmacol. 2000 Feb-Mar;55(11-12):843-52. | ||||
| 15 | Association of CYP1A1 and CYP1B1 inhibition in in vitro assays with drug-induced liver injury. J Toxicol Sci. 2021;46(4):167-176. doi: 10.2131/jts.46.167. | ||||
| 16 | Inhibitory effects of antihypertensive drugs on human cytochrome P450 2J2 activity: Potent inhibition by azelnidipine and manidipine. Chem Biol Interact. 2019 Jun 1;306:1-9. | ||||
| 17 | Comparing antihypertensive effect and plasma ciclosporin concentration between amlodipine and valsartan regimens in hypertensive renal transplant patients receiving ciclosporin therapy. Am J Cardiovasc Drugs. 2011 Dec 1;11(6):401-9. doi: 10.2165/11593800-000000000-00000. | ||||
| 18 | Role of transforming growth factor-beta1 in the progression of chronic allograft nephropathy. Nephrol Dial Transplant. 2001;16 Suppl 1:114-6. doi: 10.1093/ndt/16.suppl_1.114. | ||||
| 19 | Anastassiades CJ "Nifedipine and beta-blocker drugs." Br Med J 281 (1980): 1251-2. [PMID: 6107167] | ||||
| 20 | Canadian Pharmacists Association. | ||||
| 21 | Product Information. Tibsovo (ivosidenib). Agios Pharmaceuticals, Cambridge, MA. | ||||
| 22 | Dutreix C, Munarini F, Lorenzo S, Roesel J, Wang Y "Investigation into CYP3A4-mediated drug-drug interactions on midostaurin in healthy volunteers." Cancer Chemother Pharmacol 72 (2013): 1223-34. [PMID: 24085261] | ||||
| 23 | Product Information. Mycobutin (rifabutin). Pharmacia and Upjohn, Kalamazoo, MI. | ||||
| 24 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 25 | Benami M, Giladi Y, Shalev E "The combination of magnesium sulphate and nifedipine - a cause of neuromuscular blockade." Br J Obstet Gynaecol 101 (1994): 262-3. [PMID: 8193107] | ||||
| 26 | Hamann SR, Blouin RA, Chang SL, et al "Effects of hemodynamic changes on the elimination kinetics of verapamil and nifedipine." J Pharmacol Exp Ther 231 (1984): 301-5. [PMID: 6491984] | ||||
| 27 | Aronowitz JS, Chakos MH, Safferman AZ, Lieberman JA "Syncope associated with the combination of clozapine and enalapril." J Clin Psychopharmacol 14 (1994): 429-30. [PMID: 7884028] | ||||
| 28 | Hedaya MA, El-Afify DR, El-Maghraby GM "The effect of ciprofloxacin and clarithromycin on sildenafil oral bioavailability in human volunteers." Biopharm Drug Dispos 27 (2006): 103-10. [PMID: 16372380] | ||||
| 29 | Heinig R, Adelmann HG, Ahr G "The effect of ketoconazole on the pharmacokinetics, pharmacodynamics and safety of nisoldipine." Eur J Clin Pharmacol 55 (1999): 57-60. [PMID: 10206086] | ||||
| 30 | Product Information. Zylo Filmtab (zileuton). Abbott Pharmaceutical, Abbott Park, IL. | ||||
| 31 | Ban TA "Drug interactions with psychoactive drugs." Dis Nerv Syst 36 (1975): 164-6. [PMID: 1116424] | ||||
| 32 | Product Information. Balversa (erdafitinib). Janssen Products, LP, Horsham, PA. | ||||
| 33 | Product Information. Tykerb (lapatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 34 | Agbin NE, Brater DC, Hall SD "Interaction of diltiazem with lovastatin and pravastatin." Clin Pharmacol Ther 61 (1997): 201. [PMID: 9797793] | ||||
| 35 | Product Information. Daurismo (glasdegib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 36 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 37 | Product Information. Kalydeco (ivacaftor). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 38 | Product Information. Polivy (polatuzumab vedotin). Genentech, South San Francisco, CA. | ||||
| 39 | Product Information. Trileptal (oxcarbazepine) Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 40 | Ahmad S "Nifedipine-phenytoin interaction." J Am Coll Cardiol 3 (1984): 1582. [PMID: 6425385] | ||||
| 41 | Product Information. Xcopri (cenobamate). SK Life Science, Inc., Paramus, NJ. | ||||
| 42 | Bahls FH, Ozuna J, Ritchie DE "Interactions between calcium channel blockers and the anticonvulsants carbamazepine and phenytoin." Neurology 41 (1991): 740-2. [PMID: 2027492] | ||||
| 43 | Product Information. Tazverik (tazemetostat). Epizyme, Inc, Cambridge, MA. | ||||
| 44 | Product Information. Enablex (darifenacin). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 45 | Henry M, Kay MM, Viccellio P "Cardiogenic shock associated with calcium-channel and beta blockers: reversal with intravenous calcium chloride." Am J Emerg Med 3 (1985): 334-6. [PMID: 2860911] | ||||
| 46 | Ausband SC, Goodman PE "An unusual case of clarithromycin associated ergotism." J Emerg Med 4 (2001): 411-3. [PMID: 11728770] | ||||
| 47 | Product Information. Incivek (telaprevir). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 48 | Product Information. Olysio (simeprevir). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 49 | Product Information. Pifeltro (doravirine). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 50 | Product Information. Rescriptor (delavirdine). Pharmacia and Upjohn, Kalamazoo, MI. | ||||
| 51 | Product Information. Sustiva (efavirenz). DuPont Pharmaceuticals, Wilmington, DE. | ||||
| 52 | Product Information. Intelence (etravirine). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 53 | Product Information. Reyataz (atazanavir). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 54 | Product Information. Zocor (simvastatin). Merck & Co, Inc, West Point, PA. | ||||
| 55 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 56 | Product Information. Vaprisol (conivaptan). Cumberland Pharmaceuticals Inc, Nashville, TN. | ||||
| 57 | Product Information. Zurampic (lesinurad). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 58 | Product Information. Caplyta (lumateperone). Intra-Cellular Therapies, Inc., New York, NY. | ||||
| 59 | Blakely KM, Drucker AM, Rosen CF "Drug-induced photosensitivity-an update: Culprit drugs, prevention and management." Drug Saf 42 (2019): 827-47. [PMID: 30888626] | ||||
| 60 | Product Information. Zepzelca (lurbinectedin). Jazz Pharmaceuticals, Palo Alto, CA. | ||||
| 61 | Product Information. Lorbrena (lorlatinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 62 | Product Information. Gavreto (pralsetinib). Blueprint Medicines Corporation, Cambridge, MA. | ||||
| 63 | Product Information. Calquence (acalabrutinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 64 | Product Information. Koselugo (selumetinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 65 | Product Information. Braftovi (encorafenib). Array BioPharma Inc., Boulder, CO. | ||||
| 66 | Product Information. Ubrelvy (ubrogepant). Allergan Inc, Irvine, CA. | ||||
| 67 | Product Information. Exjade (deferasirox). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 68 | Product Information. Gilenya (fingolimod). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 69 | Product Information. Zeposia (ozanimod). Celgene Corporation, Summit, NJ. | ||||
| 70 | Product Information. Tasigna (nilotinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 71 | Doherty MM, Charman WN "The mucosa of the small intestine: how clinically relevant as an organ of drug metabolism?" Clin Pharmacokinet 41 (2002): 235-53. [PMID: 11978143] | ||||
| 72 | Product Information. Rozlytrek (entrectinib). Genentech, South San Francisco, CA. | ||||
| 73 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 74 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 75 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 76 | Francis H, Tyndall A, Webb J "Severe vascular spasm due to erythromycin-ergotamine interaction." Clin Rheumatol 3 (1984): 243-6. [PMID: 6236021] | ||||
| 77 | Product Information. Zokinvy (lonafarnib). Eiger BioPharmaceuticals, Palo Alto, CA. | ||||
| 78 | Product Information. Nubeqa (darolutamide). Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ. | ||||
| 79 | Product Information. Tracleer (bosentan). Acetelion Pharmaceuticals US, Inc, South San Francisco, CA. | ||||
| 80 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
| 81 | Product Information. Clozaril (clozapine). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
