Details of the Drug
General Information of Drug (ID: DMAI7ZV)
| Drug Name |
Diltiazem
|
|||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Acalix; Adizem; Aldizem; Anoheal; Cardil; Cardizem; Citizem; Dilacor; Dilcontin; Dilren; Diltia; Diltiazemum; Dilticard; Diltzac; Dilzen; Endrydil; Viazem; Cardizen LA; Incoril AP; Tiazac Tildiem; Tiazac XC; Adizem (TN); Altiazem (TN); Angiozem (TN); Angizem (TN); Angizem CD (TN); Apo-Diltiaz; Cardizem (Hydrochloride); Cardizem (TN); Cartia XT (TN); Dilacor-XR; Dilatam (TN); Dilatem (TN); Dilcardia (TN); Dilcontin SR (TN); Dilt-cd; Dilta-Hexal; Diltelan (TN); Diltiazem (INN); Diltiazem [INN:BAN]; Diltiazemum [INN-Latin]; Diltime (TN); Dilzem (TN); Dyalec (TN); Filazem (TN); Herben (TN); Nu-Diltiaz; Progor (TN); RG 83606 (Hydrochloride); Surazem (TN); Tiamate (TN); Tiazac (TN); Tiazac XC (TN); Tildiem (TN); Vasmulax (TN); Viazem (TN); Zandil (TN); Zemtrial (TN); D-cis-Diltiazem; MK-793 (Malate); [(2S,3S)-5-(2-dimethylaminoethyl)-2-(4-methoxyphenyl)-4-oxo-2,3-dihydro-1,5-benzothiazepin-3-yl] acetate; Acetic acid (2S,3S)-5-(2-dimethylamino-ethyl)-2-(4-methoxy-phenyl)-4-oxo-2,3,4,5-tetrahydro-benzo[b][1,4]thiazepin-3-yl ester; (+)-cis-5-[2-(dimethylamino)ethyl]-2,3-dihydro-3-hydroxy-2-(p-methoxyphenyl)-1,5-benzothiazepin-4(5H)-one acetate ester; (2S,3S)-5-(2-(dimethylamino)ethyl)-2-(4-methoxyphenyl)-4-oxo-2,3,4,5-tetrahydrobenzo[b][1,4]thiazepin-3-yl acetate; (2S,3S)-5-[2-(dimethylamino)ethyl]-2-(4-methoxyphenyl)-4-oxo-2,3,4,5-tetrahydro-1,5-benzothiazepin-3-yl acetate; (2S,3S)-5-[2-(dimethylamino)ethyl]-2-[4-(methyloxy)phenyl]-4-oxo-2,3,4,5-tetrahydro-1,5-benzothiazepin-3-yl acetate; (2S-cis)-3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-1,5-benzothiazepin-4(5H)-one
|
|||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antihypertensive Agents
|
|||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||
| Structure |
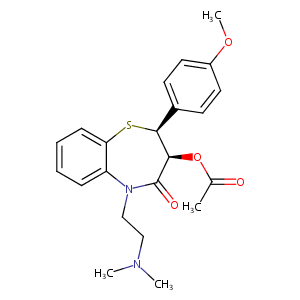 |
|||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 414.5 | ||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.1 | |||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | |||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | |||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | |||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Angina pectoris | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | BA40 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Diltiazem
Coadministration of a Drug Treating the Disease Different from Diltiazem (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Diltiazem FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2298). | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | CARDIZEM? LA (diltiazem hydrochloride) extended-release tablets - FDA Label | ||||
| 5 | Diltiazem Hydrochloride Injection Label - Bedford Laboratories | ||||
| 6 | Authors unspecified: Doxepin . | ||||
| 7 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 8 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 9 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 10 | Egr-1, the potential target of calcium channel blockers in cardioprotection with ischemia/reperfusion injury in rats. Cell Physiol Biochem. 2009;24(1-2):17-24. | ||||
| 11 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | ||||
| 12 | Comparative metabolic capabilities of CYP3A4, CYP3A5, and CYP3A7. Drug Metab Dispos. 2002 Aug;30(8):883-91. | ||||
| 13 | Drug interactions with calcium channel blockers: possible involvement of metabolite-intermediate complexation with CYP3A. Drug Metab Dispos. 2000 Feb;28(2):125-30. | ||||
| 14 | Effects of CYP3A4 inhibition by diltiazem on pharmacokinetics and dynamics of diazepam in relation to CYP2C19 genotype status. Drug Metab Dispos. 2001 Oct;29(10):1284-9. | ||||
| 15 | Significant impacts of CYP3A4*1G and CYP3A5*3 genetic polymorphisms on the pharmacokinetics of diltiazem and its main metabolites in Chinese adult kidney transplant patients. J Clin Pharm Ther. 2016 Jun;41(3):341-7. | ||||
| 16 | Role of CYP3A4 in human hepatic diltiazem N-demethylation: inhibition of CYP3A4 activity by oxidized diltiazem metabolites. J Pharmacol Exp Ther. 1997 Jul;282(1):294-300. | ||||
| 17 | FDA Drug Development and Drug Interactions | ||||
| 18 | A common 1-adrenergic receptor polymorphism predicts favorable response to rate-control therapy in atrial fibrillation. J Am Coll Cardiol. 2012 Jan 3;59(1):49-56. doi: 10.1016/j.jacc.2011.08.061. | ||||
| 19 | Anastassiades CJ "Nifedipine and beta-blocker drugs." Br Med J 281 (1980): 1251-2. [PMID: 6107167] | ||||
| 20 | Canadian Pharmacists Association. | ||||
| 21 | Abernethy DR, Egan JM, Dickinson TH, Carrum G "Substrate-selective inhibition by verapamil and diltiazem: differential disposition of antipyrine and theophylline in humans." J Pharmacol Exp Ther 244 (1988): 994-9. [PMID: 3252045] | ||||
| 22 | Francis H, Tyndall A, Webb J "Severe vascular spasm due to erythromycin-ergotamine interaction." Clin Rheumatol 3 (1984): 243-6. [PMID: 6236021] | ||||
| 23 | Bidstrup TB, Bjornsdottir I, Sidelmann UG, Thomsen MS, Hansen KT "CYP2C8 and CYP3A4 are the principal enzymes involved in the human in vitro biotransformation of the insulin secretagogue repaglinide." Br J Clin Pharmacol 56 (2003): 305-14. [PMID: 12919179] | ||||
| 24 | Product Information. Actos (pioglitazone) Takeda Pharmaceuticals America, Lincolnshire, IL. | ||||
| 25 | Product Information. Tibsovo (ivosidenib). Agios Pharmaceuticals, Cambridge, MA. | ||||
| 26 | Dutreix C, Munarini F, Lorenzo S, Roesel J, Wang Y "Investigation into CYP3A4-mediated drug-drug interactions on midostaurin in healthy volunteers." Cancer Chemother Pharmacol 72 (2013): 1223-34. [PMID: 24085261] | ||||
| 27 | Multum Information Services, Inc. Expert Review Panel. | ||||
| 28 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 29 | Borcherding SM, Baciewicz AM, Self TH "Update on rifampin drug interactions." Arch Intern Med 152 (1992): 711-6. [PMID: 1558427] | ||||
| 30 | Lamberg TS, Kivisto KT, Neuvonen PJ "Effects of verapamil and diltiazem on the pharmacokinetics and pharmacodynamics of buspirone." Clin Pharmacol Ther 63 (1998): 640-5. [PMID: 9663178] | ||||
| 31 | Lledo P, Abrams SM, Johnston A, Patel M, Pearson RM, Turner P. Influence of debrisoquine hydroxylation phenotype on the pharmacokinetics of mexiletine. Eur J Clin Pharmacol. 1993;44(1):63-67. [PMID: 8436157] | ||||
| 32 | Aronowitz JS, Chakos MH, Safferman AZ, Lieberman JA "Syncope associated with the combination of clozapine and enalapril." J Clin Psychopharmacol 14 (1994): 429-30. [PMID: 7884028] | ||||
| 33 | Heinig R, Adelmann HG, Ahr G "The effect of ketoconazole on the pharmacokinetics, pharmacodynamics and safety of nisoldipine." Eur J Clin Pharmacol 55 (1999): 57-60. [PMID: 10206086] | ||||
| 34 | Bolland MJ, Bagg W, Thomas MG, Lucas JA, Ticehurst R, Black PN "Cushing's syndrome due to interaction between inhaled corticosteroids and itraconazole." Ann Pharmacother 38 (2004): 46-9. [PMID: 14742792] | ||||
| 35 | Product Information. Zylo Filmtab (zileuton). Abbott Pharmaceutical, Abbott Park, IL. | ||||
| 36 | Product Information. Synercid (dalfopristin-quinupristin) Rhone-Poulenc Rorer, Collegeville, PA. | ||||
| 37 | Product Information. Ketek (telithromycin). Aventis Pharmaceuticals, Bridgewater, NJ. | ||||
| 38 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 39 | Adachi Y, Suzuki H, Sugiyama Y "Quantitative evaluation of the function of small intestinal P-glycoprotein: comparative studies between in Situ and in Vivo." Pharm Res 20 (2003): 1163-9. [PMID: 12948013] | ||||
| 40 | Product Information. Talzenna (talazoparib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 41 | Product Information. Ixempra (ixabepilone). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 42 | Product Information. Tykerb (lapatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 43 | Abbas R, Hug BA, Leister C, Burns J, Sonnichsen D "Pharmacokinetics of oral neratinib during co-administration of ketoconazole in healthy subjects." Br J Clin Pharmacol 71 (2011): 522-7. [PMID: 21395644] | ||||
| 44 | Product Information. Verzenio (abemaciclib). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 45 | Product Information. Tukysa (tucatinib). Seattle Genetics Inc, Bothell, WA. | ||||
| 46 | Product Information. Jevtana (cabazitaxel). sanofi-aventis , Bridgewater, NJ. | ||||
| 47 | Product Information. Fareston (toremifene). Schering Laboratories, Kenilworth, NJ. | ||||
| 48 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 49 | Product Information. Raxar (grepafloxacin). Glaxo Wellcome, Research Triangle Park, NC. | ||||
| 50 | Agbin NE, Brater DC, Hall SD "Interaction of diltiazem with lovastatin and pravastatin." Clin Pharmacol Ther 61 (1997): 201. [PMID: 9797793] | ||||
| 51 | Product Information. Opsumit (macitentan). Actelion Pharmaceuticals US Inc, South San Francisco, CA. | ||||
| 52 | Product Information. Daurismo (glasdegib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 53 | Product Information. Arcapta Neohaler (indacaterol). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 54 | Product Information. Dificid (fidaxomicin). Optimer Pharmaceuticals, San Diego, CA. | ||||
| 55 | Warrington SJ, Ankier SI, Turner P "Evaluation of possible interactions between ethanol and trazodone or amitriptyline." Neuropsychobiology 15 (1986): 31-7. [PMID: 3725002] | ||||
| 56 | Renton KW "Inhibition of hepatic microsomal drug metabolism by the calcium channel blockers diltiazem and verapamil." Biochem Pharmacol 34 (1985): 2549-53. [PMID: 4015695] | ||||
| 57 | Product Information. Korlym (mifepristone). Corcept Therapeutics Incorporated, Menlo Park, CA. | ||||
| 58 | Product Information. Prevymis (letermovir). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 59 | Product Information. Xarelto (rivaroxaban). Bayer Inc, Toronto, IA. | ||||
| 60 | Product Information. Emend (aprepitant). Merck & Company Inc, West Point, PA. | ||||
| 61 | Product Information. Viibryd (vilazodone). Trovis Pharmaceuticals LLC, New Haven, CT. | ||||
| 62 | Product Information. Serzone (nefazodone). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 63 | Ban TA "Drug interactions with psychoactive drugs." Dis Nerv Syst 36 (1975): 164-6. [PMID: 1116424] | ||||
| 64 | Product Information. Celexa (citalopram). Forest Pharmaceuticals, St. Louis, MO. | ||||
| 65 | Product Information. Rexulti (brexpiprazole). Otsuka American Pharmaceuticals Inc, Rockville, MD. | ||||
| 66 | Okubo M, Murayama N, Miura J, Chiba Y, Yamazaki H "Effects of cytochrome P450 2D6 and 3A5 genotypes and possible coadministered medicines on the metabolic clearance of antidepressant mirtazapine in Japanese patients." Biochem Pharmacol 93 (2015): 104-9. [PMID: 25475885] | ||||
| 67 | Product Information. Polivy (polatuzumab vedotin). Genentech, South San Francisco, CA. | ||||
| 68 | Nakasa H, Nakamura H, Ono S, Tsutsui M, Kiuchi M, Ohmori S, Kitada M "Prediction of drug-drug interactions of zonisamide metabolism in humans from in vitro data." Eur J Clin Pharmacol 54 (1998): 177-83. [PMID: 9626925] | ||||
| 69 | Ahmad S "Nifedipine-phenytoin interaction." J Am Coll Cardiol 3 (1984): 1582. [PMID: 6425385] | ||||
| 70 | Ahmad S "Diltiazem-carbamazepine interaction." Am Heart J 120 (1990): 1485-6. [PMID: 2248204] | ||||
| 71 | Rubin AS, Zablocki AD "Hyperkalemia, verapamil, and dantrolene." Anesthesiology 66 (1987): 246-9. [PMID: 3813090] | ||||
| 72 | Product Information. Aliqopa (copanlisib). Bayer Pharmaceutical Inc, West Haven, CT. | ||||
| 73 | Product Information. Tazverik (tazemetostat). Epizyme, Inc, Cambridge, MA. | ||||
| 74 | Product Information. VESIcare (solifenacin). GlaxoSmithKline, Research Triangle Park, NC. | ||||
| 75 | Product Information. Myrbetriq (mirabegron). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 76 | Brynne N, Forslund C, Hallen B, Gustafsson LL, Bertilsson L "Ketoconazole inhibits the metabolism of tolterodine in subjects with deficient CYP2D6 activity." Br J Clin Pharmacol 48 (1999): 564-72. [PMID: 10583027] | ||||
| 77 | Agencia Espaola de Medicamentos y Productos Sanitarios Healthcare "Centro de informacion online de medicamentos de la AEMPS - CIMA.". | ||||
| 78 | Bedford TA, Rowbotham DJ "Cisapride: drug interactions of clinical significance." Drug Saf 15 (1996): 167-75. [PMID: 8879971] | ||||
| 79 | Packer M "Combined beta-adrenergic and calcium-entry blockage in angina pectoris." N Engl J Med 320 (1989): 709-18. [PMID: 2564164] | ||||
| 80 | Akdag I, Ersoy A, Kahvecioglu S, Gullulu M, Dilek K "Acute colchicine intoxication during clarithromycin administration in patients with chronic renal failure." J Nephrol 19 (2006): 515-7. [PMID: 17048210] | ||||
| 81 | Product Information. Inspra (eplerenone). Searle, Chicago, IL. | ||||
| 82 | Andrejak M, Hary L, Andrejak MT, Lesbre JP "Diltiazem increases steady state digoxin serum levels in patients with cardiac disease." J Clin Pharmacol 27 (1987): 967-70. [PMID: 3437068] | ||||
| 83 | Product Information. Incivek (telaprevir). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 84 | Product Information. Olysio (simeprevir). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 85 | Product Information. Mycobutin (rifabutin). Pharmacia and Upjohn, Kalamazoo, MI. | ||||
| 86 | Product Information. Adcetris (brentuximab vedotin). Seattle Genetics Inc, Bothell, WA. | ||||
| 87 | Product Information. Pifeltro (doravirine). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 88 | Product Information. Rescriptor (delavirdine). Pharmacia and Upjohn, Kalamazoo, MI. | ||||
| 89 | Product Information. Agenerase (amprenavir). Glaxo Wellcome, Research Triangle Pk, NC. | ||||
| 90 | Product Information. Rukobia (fostemsavir). ViiV Healthcare, Research Triangle Park, NC. | ||||
| 91 | Product Information. Stribild (cobicistat/elvitegravir/emtricitabine/tenofov). Gilead Sciences, Foster City, CA. | ||||
| 92 | Product Information. Aptivus (tipranavir). Boehringer-Ingelheim, Ridgefield, CT. | ||||
| 93 | Product Information. Tivicay (dolutegravir). ViiV Healthcare, Research Triangle Park, NC. | ||||
| 94 | Product Information. Intelence (etravirine). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 95 | Product Information. Prezista (darunavir). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 96 | Product Information. Selzentry (maraviroc). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 97 | Olkkola KT, Palkama VJ, Neuvonen PJ "Ritonavir's role in reducing fentanyl clearance and prolonging its half-life." Anesthesiology 91 (1999): 681-5. [PMID: 10485779] | ||||
| 98 | Product Information. Vaprisol (conivaptan). Cumberland Pharmaceuticals Inc, Nashville, TN. | ||||
| 99 | Product Information. Samsca (tolvaptan). Otsuka American Pharmaceuticals Inc, Rockville, MD. | ||||
| 100 | Product Information. Altabax (retapamulin topical). GlaxoSmithKline, Research Triangle Park, NC. | ||||
| 101 | Product Information. Orladeyo (berotralstat). BioCryst Pharmaceuticals Inc, Durham, NC. | ||||
| 102 | Product Information. Belsomra (suvorexant). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 103 | Alderman CP, Gebauer MG, Gilbert AL, Condon JT "Possible interaction of zopiclone and nefazodone." Ann Pharmacother 35 (2001): 1378-80. [PMID: 11724087] | ||||
| 104 | Backman JT, Olkkola KT, Aranko K, Himberg JJ, Neuvonen PJ "Dose of midazolam should be reduced during diltiazem and verapamil treatments." Br J Clin Pharmacol 37 (1994): 221-5. [PMID: 8198928] | ||||
| 105 | Product Information. Hetlioz (tasimelteon). Vanda Pharmaceuticals Inc, Rockville, MD. | ||||
| 106 | Product Information. Caplyta (lumateperone). Intra-Cellular Therapies, Inc., New York, NY. | ||||
| 107 | Farkas D, Volak L, Harmatz J, von Moltke L, Court M, Greenblatt D "Short-term clarithromycin administration impairs clearance and enhances pharmacodynamic effects of trazodone but not of zolpidem." Clin Pharmacol Ther 85 (2009): 644-50. [PMID: 19242403] | ||||
| 108 | Blakely KM, Drucker AM, Rosen CF "Drug-induced photosensitivity-an update: Culprit drugs, prevention and management." Drug Saf 42 (2019): 827-47. [PMID: 30888626] | ||||
| 109 | Product Information. Zepzelca (lurbinectedin). Jazz Pharmaceuticals, Palo Alto, CA. | ||||
| 110 | Product Information. Tagrisso (osimertinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 111 | Product Information. Gavreto (pralsetinib). Blueprint Medicines Corporation, Cambridge, MA. | ||||
| 112 | Product Information. Tabrecta (capmatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 113 | Abernethy DR, Wesche DL, Barbey JT, et al. "Stereoselective halofantrine disposition and effect: concentration-related QTc prolongation." Br J Clin Pharmacol 51 (2001): 231-7. [PMID: 11298069] | ||||
| 114 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 115 | Product Information. Copiktra (duvelisib). Verastem, Inc., Needham, MA. | ||||
| 116 | Product Information. Imbruvica (ibrutinib). Pharmacyclics Inc, Sunnyvale, CA. | ||||
| 117 | Product Information. Iclusig (ponatinib). Ariad Pharmaceuticals Inc, Cambridge, MA. | ||||
| 118 | Product Information. Koselugo (selumetinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 119 | Product Information. Braftovi (encorafenib). Array BioPharma Inc., Boulder, CO. | ||||
| 120 | Product Information. Ubrelvy (ubrogepant). Allergan Inc, Irvine, CA. | ||||
| 121 | Product Information. Nurtec ODT (rimegepant). Biohaven Pharmaceuticals, New Haven, CT. | ||||
| 122 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 123 | Hamberg P, Woo MM, Chen LC, et al. "Effect of ketoconazole-mediated CYP3A4 inhibition on clinical pharmacokinetics of panobinostat (LBH589), an orally active histone deacetylase inhibitor." Cancer Chemother Pharmacol 68 (2011): 805-13. [PMID: 21706316] | ||||
| 124 | Product Information. Gilenya (fingolimod). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 125 | Product Information. Zeposia (ozanimod). Celgene Corporation, Summit, NJ. | ||||
| 126 | Product Information. Istodax (romidepsin). Gloucester Pharmaceuticals, Cambridge, MA. | ||||
| 127 | Product Information. Tasigna (nilotinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 128 | Product Information. Gleevec (imatinib mesylate). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 129 | Product Information. Sprycel (dasatinib). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 130 | Product Information. Meridia (sibutramine). Knoll Pharmaceutical Company, Whippany, NJ. | ||||
| 131 | Product Information. Orlaam (levomethadyl acetate) Roxanne Laboratories Inc, Columbus, OH. | ||||
| 132 | Product Information. Bextra (valdecoxib). Pharmacia Corporation, Peapack, NJ. | ||||
| 133 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 134 | Product Information. Zejula (niraparib). Tesaro Inc., Waltham, MA. | ||||
| 135 | Product Information. Butorphanol Tartrate (butorphanol). Apotex Corporation, Weston, FL. | ||||
| 136 | Product Information. Buprenex (buprenorphine). Reckitt and Colman Pharmaceutical, Richmond, VA. | ||||
| 137 | Hutchinson MR, Menelaou A, Foster DJ, Coller JK, Somogyi AA "CYP2D6 and CYP3A4 involvement in the primary oxidative metabolism of hydrocodone by human liver microsomes." Br J Clin Pharmacol 57 (2004): 287-97. [PMID: 14998425] | ||||
| 138 | Product Information. Nuplazid (pimavanserin). Accelis Pharma, East Windsor, NJ. | ||||
| 139 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 140 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 141 | Product Information. Zokinvy (lonafarnib). Eiger BioPharmaceuticals, Palo Alto, CA. | ||||
| 142 | Product Information. Xtandi (enzalutamide). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 143 | Product Information. Orgovyx (relugolix). Myovant Sciences, Inc., Brisbane, CA. | ||||
| 144 | Franco-Salinas G, de la Rosette JJ, Michel MC "Pharmacokinetics and pharmacodynamics of tamsulosin in its modified-release and oral controlled absorption system formulations." Clin Pharmacokinet 49 (2010): 177-88. [PMID: 20170206] | ||||
| 145 | Product Information. Rapaflo (silodosin). Watson Pharmaceuticals, Corona, CA. | ||||
| 146 | Product Information. Duagen (dutasteride). GlaxoSmithKline Healthcare, Pittsburgh, PA. | ||||
| 147 | Product Information. Afinitor (everolimus). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 148 | Product Information. Torisel (temsirolimus). Wyeth-Ayerst Laboratories, Philadelphia, PA. | ||||
| 149 | Product Information. Rinvoq (upadacitinib). AbbVie US LLC, North Chicago, IL. | ||||
| 150 | Product Information. Procardia (nifedipine). Pfizer US Pharmaceuticals, New York, NY. | ||||
| 151 | DeVane CL, Nemeroff CB "Clinical pharmacokinetics of quetiapine - An atypical antipsychotic." Clin Pharmacokinet 40 (2001): 509-22. [PMID: 11510628] | ||||
| 152 | Product Information. Stendra (avanafil). Vivus Inc, Mountain View, CA. | ||||
| 153 | Product Information. Cialis (tadalafil). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 154 | Product Information. Oxbryta (voxelotor). Global Blood Therapeutics, Inc., South San Francisco, CA. | ||||
| 155 | Product Information. Odomzo (sonidegib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 156 | Aronson JK, Grahame-Smith DG "Clinical pharmacology: adverse drug interactions." Br Med J 282 (1981): 288-91. [PMID: 6779990] | ||||
| 157 | Bergmann TK, Filppula AM, Launiainen T, Nielsen F, Backman J, Brosen K "Neurotoxicity and low paclitaxel clearance associated with concomitant clopidogrel therapy in a 60 year old Caucasian woman with ovarian carcinoma." Br J Clin Pharmacol (2015):. [PMID: 26446447] | ||||
| 158 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
| 159 | Product Information. Cometriq (cabozantinib). Exelixis Inc, S San Francisco, CA. | ||||
| 160 | Cakaloglu Y, Tredger JM, Devlin J, Williams R "Importance of cytochrome p-450IIIA activity in determining dosage and blood levels of FK 506 and cyclosporine in liver transplant recipients." Hepatology 20 (1994): 309-16. [PMID: 7519161] | ||||
| 161 | Product Information. Orilissa (elagolix). AbbVie US LLC, North Chicago, IL. | ||||
| 162 | Product Information. Clozaril (clozapine). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 163 | Product Information. Rythmol SR (propafenone). GlaxoSmithKline, Research Triangle Park, NC. | ||||
| 164 | Kerin NZ, Aragon E, Faitel K, Frumin H, Rubenfire M "Long-term efficacy and toxicity of high- and low-dose amiodarone regimens." J Clin Pharmacol 29 (1989): 418-23. [PMID: 2661600] | ||||
