Details of the Drug
General Information of Drug (ID: DM2AQ5N)
| Drug Name |
Spironolactone
|
||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Abbolactone; Acelat; Aldace; Aldactazide; Aldactide; Aldactone; Alderon; Aldopur; Almatol; Altex; Aquareduct; Deverol; Diatensec; Dira; Duraspiron; Espironolactona; Euteberol; Flumach; Frumikal; Jenaspiron; Lacalmin; Lacdene; Laractone; Melarcon; Nefurofan; NovoSpiroton; Osyrol; Practon; SNL; Sagisal; Sincomen; Spiractin; Spiresis; Spiretic; Spiridon; Spirobeta; Spiroctan; Spiroctanie; Spiroderm; Spirogamma; Spirolactone; Spirolakton; Spirolang; Spirolone; Spirone; Spironocompren; Spironolactonum; Spironolattone; Spironone; Spirospare; Sprioderm; Suracton; Uractone; Urusonin; Veroshpiron; Verospiron; Verospirone; Xenalon; Aldactone A; Alphapharm Brand of Spironolactone; Alpharma Brand of Spironolactone; Alter Brand of Spironolactone; Ashbourne Brand of Spironolactone; Azupharma Brand of Spironolactone; Betapharm Brand of Spironolactone; Cardel Brand of Spironolactone; Ct Arzneimittel Brand of Spironolactone; Dexo Brand of Spironolactone; Espironolactona Alter; Espironolactona Mundogen; Generosan Brand of Spironolactone; Hormosan Brand of Spironolactone; Jenapharm Brand of Spironolactone; Merck dura Brand of Spironolactone; Mundogen Brand of Spironolactone; Novo Spiroton; Novopharm Brand of Spironolactone; Pfizer Brand of Spironolactone; Pharmafrid Brand of Spironolactone; Roche Brand of Spironolactone; Searle Brand of Spironolactone; Spiro von ct; Spirono Isis; Spironolactone A; Spironolattone [DCIT]; Verospirone Opianin; Worwag Brand of Spironolactone; LT00772287; SC 9420; SC9420; Aldactazide (TN); Aldactone (TN); Berlactone (TN); Ct-Arzneimittel Brand of Spironolactone; Espironolactona [INN-Spanish]; Mayoly-Spindler Brand of Spironolactone; Novo-Spiroton; SC-9420; Spiractin (TN); Spiro-Tablinen; Spirono-Isis; Spironolactonum [INN-Latin]; Spirotone (TN); Supra-puren; Verospiron (TN); Von ct, spiro; Novo-Spiroton (TN); Spironolactone [BAN:INN:JAN]; Spironolactone [INN:BAN:JAN]; Spiro L.U.T.; Spironolactone (JP15/USP/INN); Spiro[17H-cyclopenta[a]phenauthrene-17,2'-(3'H)-furan]; Spiro(17H-cyclopenta(a)phenauthrene-17,2'-(3'H)-furan); 4-Pregnen-21-oic acid-17alpha-ol-3-one-7alpha-thiol gamma-lactone 7-acetate; 7-alpha-Acetylthio-3-oxo-17-alpha-pregn-4-ene-21,17-beta-carbolactone; 7alpha-(acetylsulfanyl)-3-oxo-17alpha-pregn-4-ene-21,17-carbolactone
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Diuretics
|
||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
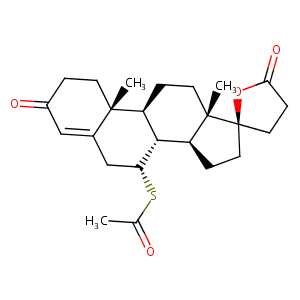 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 416.6 | |||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.9 | ||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Chronic heart failure | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | BD1Z | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Spironolactone
Coadministration of a Drug Treating the Disease Different from Spironolactone (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Spironolactone FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2875). | ||||
| 3 | Spironolactone in Covid-19 Induced ARDS | ||||
| 4 | BDDCS applied to over 900 drugs | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | ||||
| 7 | Comparison of the Hershberger assay and androgen receptor binding assay of twelve chemicals. Toxicology. 2004 Feb 15;195(2-3):177-86. doi: 10.1016/j.tox.2003.09.012. | ||||
| 8 | Amiloride, spironolactone, and potassium chloride in thiazide-treated hypertensive patients. Clin Pharmacol Ther. 1980 Apr;27(4):533-43. doi: 10.1038/clpt.1980.75. | ||||
| 9 | Pregnane X receptor mediates the induction of P-glycoprotein by spironolactone in HepG2 cells. Toxicology. 2011 Jul 11;285(1-2):18-24. doi: 10.1016/j.tox.2011.03.015. Epub 2011 Apr 1. | ||||
| 10 | Early identification of clinically relevant drug interactions with the human bile salt export pump (BSEP/ABCB11). Toxicol Sci. 2013 Dec;136(2):328-43. | ||||
| 11 | Palmitate increases the susceptibility of cells to drug-induced toxicity: an in vitro method to identify drugs with potential contraindications in patients with metabolic disease. Toxicol Sci. 2012 Oct;129(2):346-62. doi: 10.1093/toxsci/kfs208. Epub 2012 Jun 14. | ||||
| 12 | Effect of spironolactone on plasma brain natriuretic peptide and left ventricular remodeling in patients with congestive heart failure. J Am Coll Cardiol. 2001 Apr;37(5):1228-33. doi: 10.1016/s0735-1097(01)01116-0. | ||||
| 13 | Examination of 209 drugs for inhibition of cytochrome P450 2C8. J Clin Pharmacol. 2005 Jan;45(1):68-78. | ||||
| 14 | Receptor-dependent regulation of the CYP3A4 gene. Toxicology. 2002 Dec 27;181-182:199-202. | ||||
| 15 | Spironolactone induces apoptosis in human mononuclear cells. Association between apoptosis and cytokine suppression. Apoptosis. 2006 Apr;11(4):573-9. doi: 10.1007/s10495-006-4919-3. | ||||
| 16 | Marcy TR, Ripley TL "Aldosterone antagonists in the treatment of heart failure." Am J Health Syst Pharm 63 (2006): 49-58. [PMID: 16373465] | ||||
| 17 | Dean S, Kendall MJ, Potter S, Thompson MH, Jackson DA "Nadolol in combination with indapamide and xipamide in resistant hypertensives." Eur J Clin Pharmacol 28 (1985): 29-33. [PMID: 3987783] | ||||
| 18 | Product Information. Aldactone (spironolactone). Searle, Skokie, IL. | ||||
| 19 | Multum Information Services, Inc. Expert Review Panel. | ||||
| 20 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 21 | Ban TA "Drug interactions with psychoactive drugs." Dis Nerv Syst 36 (1975): 164-6. [PMID: 1116424] | ||||
| 22 | Aronowitz JS, Chakos MH, Safferman AZ, Lieberman JA "Syncope associated with the combination of clozapine and enalapril." J Clin Psychopharmacol 14 (1994): 429-30. [PMID: 7884028] | ||||
| 23 | Adachi Y, Suzuki H, Sugiyama Y "Quantitative evaluation of the function of small intestinal P-glycoprotein: comparative studies between in Situ and in Vivo." Pharm Res 20 (2003): 1163-9. [PMID: 12948013] | ||||
| 24 | Product Information. Talzenna (talazoparib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 25 | Agencia Espaola de Medicamentos y Productos Sanitarios Healthcare "Centro de informacion online de medicamentos de la AEMPS - CIMA.". | ||||
| 26 | Muller FO, Schall R, Devaal AC, Groenewoud G, Hundt HKL, Middle MV "Influence of meloxicam on furosemide pharmacokinetics and pharmacodynamics in healthy volunteers." Eur J Clin Pharmacol 48 (1995): 247-51. [PMID: 7589049] | ||||
| 27 | Product Information. Savella (milnacipran). Forest Pharmaceuticals, St. Louis, MO. | ||||
| 28 | Product Information. Dificid (fidaxomicin). Optimer Pharmaceuticals, San Diego, CA. | ||||
| 29 | Warrington SJ, Ankier SI, Turner P "Evaluation of possible interactions between ethanol and trazodone or amitriptyline." Neuropsychobiology 15 (1986): 31-7. [PMID: 3725002] | ||||
| 30 | Product Information. Yasmin (drospirenone-ethinyl estradiol) Berlex Laboratories, Richmond, CA. | ||||
| 31 | Yassa R, Nastase C, Camille Y, Henderson M, Belzile L, Beland F "Carbamazepine, diuretics, and hyponatremia: a possible interaction." J Clin Psychiatry 48 (1987): 281-3. [PMID: 3597330] | ||||
| 32 | Product Information. Aptiom (eslicarbazepine). Sunovion Pharmaceuticals Inc, Marlborough, MA. | ||||
| 33 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 34 | Product Information. Adcetris (brentuximab vedotin). Seattle Genetics Inc, Bothell, WA. | ||||
| 35 | Product Information. Rukobia (fostemsavir). ViiV Healthcare, Research Triangle Park, NC. | ||||
| 36 | Product Information. Midamor (amiloride). Merck & Co, Inc, West Point, PA. | ||||
| 37 | Product Information. Tekturna (aliskiren). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 38 | Jarman PR, Mather HM "Diabetes may be independent risk factor for hyperkalaemia." BMJ 327 (2003): 812. [PMID: 14525902] | ||||
| 39 | McNay JL, Oran E "Possible predisposition of diabetic patients to hyperkalemia following administration of potassium-retaining diuretic, amiloride (MK 870)." Metabolism 19 (1970): 58-70. [PMID: 5410663] | ||||
| 40 | Product Information. Samsca (tolvaptan). Otsuka American Pharmaceuticals Inc, Rockville, MD. | ||||
| 41 | Product Information. Orladeyo (berotralstat). BioCryst Pharmaceuticals Inc, Durham, NC. | ||||
| 42 | Product Information. Ubrelvy (ubrogepant). Allergan Inc, Irvine, CA. | ||||
| 43 | Product Information. Istodax (romidepsin). Gloucester Pharmaceuticals, Cambridge, MA. | ||||
| 44 | Product Information. Zejula (niraparib). Tesaro Inc., Waltham, MA. | ||||
| 45 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 46 | Product Information. Orgovyx (relugolix). Myovant Sciences, Inc., Brisbane, CA. | ||||
| 47 | Product Information. Rapaflo (silodosin). Watson Pharmaceuticals, Corona, CA. | ||||
| 48 | Aronson JK, Grahame-Smith DG "Clinical pharmacology: adverse drug interactions." Br Med J 282 (1981): 288-91. [PMID: 6779990] | ||||
| 49 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 50 | Perazella MA "Drug-induced hyperkalemia: old culprits and new offenders." Am J Med 109 (2000): 307-14. [PMID: 10996582] | ||||
| 51 | Canaday DH, Johnson JR "Hyperkalemia in elderly patients receiving standard doses of trimethoprim-sulfamethoxazole." Ann Intern Med 120 (1994): 438. [PMID: 8304666] | ||||
| 52 | Product Information. Clozaril (clozapine). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
