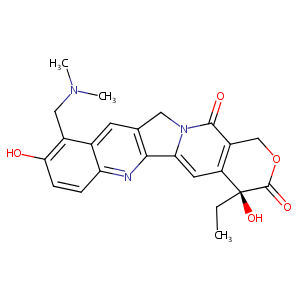
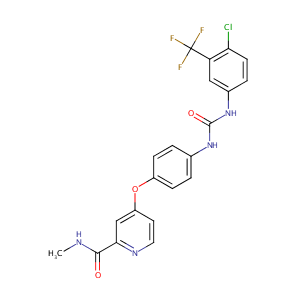
| DOT Name |
DOT ID |
UniProt ID |
Mode of Action |
REF |
|
ATP-dependent translocase ABCB1 (ABCB1)
|
OTEJROBO
|
MDR1_HUMAN
|
Increases Expression
|
[17] |
|
Broad substrate specificity ATP-binding cassette transporter ABCG2 (ABCG2)
|
OTW8V2V1
|
ABCG2_HUMAN
|
Decreases Response To Substance
|
[18] |
|
DNA topoisomerase 2-alpha (TOP2A)
|
OT6LPS08
|
TOP2A_HUMAN
|
Decreases Expression
|
[19] |
|
Catalase (CAT)
|
OTHEBX9R
|
CATA_HUMAN
|
Increases Expression
|
[20] |
|
Epidermal growth factor receptor (EGFR)
|
OTAPLO1S
|
EGFR_HUMAN
|
Decreases Expression
|
[21] |
|
Cellular tumor antigen p53 (TP53)
|
OTIE1VH3
|
P53_HUMAN
|
Increases Activity
|
[22] |
|
Transcription factor Jun (JUN)
|
OTCYBO6X
|
JUN_HUMAN
|
Increases Phosphorylation
|
[23] |
|
Poly polymerase 1 (PARP1)
|
OT310QSG
|
PARP1_HUMAN
|
Increases Cleavage
|
[24] |
|
Apoptosis regulator Bcl-2 (BCL2)
|
OT9DVHC0
|
BCL2_HUMAN
|
Decreases Expression
|
[25] |
|
Proliferating cell nuclear antigen (PCNA)
|
OTHZ1RIA
|
PCNA_HUMAN
|
Decreases Expression
|
[21] |
|
G2/mitotic-specific cyclin-B1 (CCNB1)
|
OT19S7E5
|
CCNB1_HUMAN
|
Decreases Expression
|
[26] |
|
Histone H2AX (H2AX)
|
OT18UX57
|
H2AX_HUMAN
|
Increases Expression
|
[26] |
|
Methylated-DNA--protein-cysteine methyltransferase (MGMT)
|
OT40A9WH
|
MGMT_HUMAN
|
Decreases Expression
|
[27] |
|
G1/S-specific cyclin-E1 (CCNE1)
|
OTLD7UID
|
CCNE1_HUMAN
|
Decreases Expression
|
[21] |
|
Tumor necrosis factor receptor superfamily member 6 (FAS)
|
OTP9XG86
|
TNR6_HUMAN
|
Decreases Expression
|
[25] |
|
Catenin beta-1 (CTNNB1)
|
OTZ932A3
|
CTNB1_HUMAN
|
Increases Cleavage
|
[17] |
|
Cyclin-dependent kinase inhibitor 1 (CDKN1A)
|
OTQWHCZE
|
CDN1A_HUMAN
|
Increases Expression
|
[24] |
|
Caspase-3 (CASP3)
|
OTIJRBE7
|
CASP3_HUMAN
|
Increases Activity
|
[28] |
|
Caspase-2 (CASP2)
|
OTUDYSPP
|
CASP2_HUMAN
|
Increases Cleavage
|
[28] |
|
Apoptosis regulator BAX (BAX)
|
OTAW0V4V
|
BAX_HUMAN
|
Increases Expression
|
[26] |
|
Caspase-8 (CASP8)
|
OTA8TVI8
|
CASP8_HUMAN
|
Increases Activity
|
[17] |
|
Hypoxia-inducible factor 1-alpha (HIF1A)
|
OTADSC03
|
HIF1A_HUMAN
|
Decreases Expression
|
[8] |
|
ATP-binding cassette sub-family C member 2 (ABCC2)
|
OTJSIGV5
|
MRP2_HUMAN
|
Increases Expression
|
[17] |
|
Tumor protein 63 (TP63)
|
OT0WOOKQ
|
P63_HUMAN
|
Decreases Expression
|
[26] |
|
Bcl-2-like protein 12 (BCL2L12)
|
OTS6IFZY
|
B2L12_HUMAN
|
Decreases Expression
|
[25] |
|
Three-prime repair exonuclease 1 (TREX1)
|
OTQG7K12
|
TREX1_HUMAN
|
Increases Expression
|
[23] |
|
Histone H2A.V (H2AZ2)
|
OT0I5H2P
|
H2AV_HUMAN
|
Affects Response To Substance
|
[9] |
|
Receptor-interacting serine/threonine-protein kinase 2 (RIPK2)
|
OT0KMIYQ
|
RIPK2_HUMAN
|
Affects Response To Substance
|
[9] |
|
2-oxoisovalerate dehydrogenase subunit alpha, mitochondrial (BCKDHA)
|
OT0LHOZB
|
ODBA_HUMAN
|
Affects Response To Substance
|
[9] |
|
Secreted frizzled-related protein 1 (SFRP1)
|
OT0U9G41
|
SFRP1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Tripartite motif-containing protein 2 (TRIM2)
|
OT0V1YVC
|
TRIM2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Insulin-like growth factor-binding protein 7 (IGFBP7)
|
OT1D416P
|
IBP7_HUMAN
|
Affects Response To Substance
|
[9] |
|
Tetraspanin-8 (TSPAN8)
|
OT1F68WQ
|
TSN8_HUMAN
|
Affects Response To Substance
|
[9] |
|
Tumor necrosis factor alpha-induced protein 8 (TNFAIP8)
|
OT1G9297
|
TFIP8_HUMAN
|
Affects Response To Substance
|
[9] |
|
Aldehyde oxidase (AOX1)
|
OT2FZP6H
|
AOXA_HUMAN
|
Affects Response To Substance
|
[9] |
|
ATP-binding cassette sub-family D member 3 (ABCD3)
|
OT2PITLX
|
ABCD3_HUMAN
|
Affects Response To Substance
|
[9] |
|
Optineurin (OPTN)
|
OT2UXWH9
|
OPTN_HUMAN
|
Affects Response To Substance
|
[9] |
|
Epidermal growth factor-like protein 6 (EGFL6)
|
OT331X59
|
EGFL6_HUMAN
|
Decreases Response To Substance
|
[10] |
|
Protein FAM171A1 (FAM171A1)
|
OT33BHEP
|
F1711_HUMAN
|
Affects Response To Substance
|
[9] |
|
Filamin-C (FLNC)
|
OT3F8J6Y
|
FLNC_HUMAN
|
Affects Response To Substance
|
[9] |
|
Cleavage stimulation factor subunit 2 tau variant (CSTF2T)
|
OT3IKRL2
|
CSTFT_HUMAN
|
Affects Response To Substance
|
[9] |
|
Fatty acid CoA ligase Acsl3 (ACSL3)
|
OT3MWER1
|
ACSL3_HUMAN
|
Affects Response To Substance
|
[9] |
|
Integrin alpha-5 (ITGA5)
|
OT3RCI67
|
ITA5_HUMAN
|
Affects Response To Substance
|
[9] |
|
Platelet-derived growth factor C (PDGFC)
|
OT3ZJ0XU
|
PDGFC_HUMAN
|
Affects Response To Substance
|
[9] |
|
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 4 (PIN4)
|
OT403QC1
|
PIN4_HUMAN
|
Affects Response To Substance
|
[9] |
|
C-Jun-amino-terminal kinase-interacting protein 4 (SPAG9)
|
OT45AHMB
|
JIP4_HUMAN
|
Affects Response To Substance
|
[9] |
|
High mobility group protein B1 (HMGB1)
|
OT4B7CPF
|
HMGB1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Sorcin (SRI)
|
OT4R3EAC
|
SORCN_HUMAN
|
Affects Response To Substance
|
[9] |
|
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A (PDE8A)
|
OT4RF9Z4
|
PDE8A_HUMAN
|
Affects Response To Substance
|
[9] |
|
Argininosuccinate synthase (ASS1)
|
OT4ZMG0Q
|
ASSY_HUMAN
|
Affects Response To Substance
|
[9] |
|
Polyphosphoinositide phosphatase (FIG4)
|
OT501PY9
|
FIG4_HUMAN
|
Affects Response To Substance
|
[9] |
|
Ubiquitin-like protein ISG15 (ISG15)
|
OT53QQ7N
|
ISG15_HUMAN
|
Affects Response To Substance
|
[9] |
|
RAC-gamma serine/threonine-protein kinase (AKT3)
|
OT5M2LFI
|
AKT3_HUMAN
|
Affects Response To Substance
|
[9] |
|
Leukocyte elastase inhibitor (SERPINB1)
|
OT5RDUFO
|
ILEU_HUMAN
|
Affects Response To Substance
|
[9] |
|
Collagen alpha-2(V) chain (COL5A2)
|
OT5VOSQE
|
CO5A2_HUMAN
|
Decreases Response To Substance
|
[10] |
|
Transcription activator BRG1 (SMARCA4)
|
OT68WOPQ
|
SMCA4_HUMAN
|
Affects Response To Substance
|
[9] |
|
Metal cation symporter ZIP8 (SLC39A8)
|
OT6C6MNV
|
S39A8_HUMAN
|
Affects Response To Substance
|
[9] |
|
Regulator of G-protein signaling 20 (RGS20)
|
OT6CGYHW
|
RGS20_HUMAN
|
Affects Response To Substance
|
[9] |
|
BTB/POZ domain-containing protein KCTD3 (KCTD3)
|
OT6EGR42
|
KCTD3_HUMAN
|
Affects Response To Substance
|
[9] |
|
Melanoma-associated antigen D1 (MAGED1)
|
OT6EOLFC
|
MAGD1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Ferritin heavy chain (FTH1)
|
OT6IFS0O
|
FRIH_HUMAN
|
Affects Response To Substance
|
[9] |
|
Protein-glutamine gamma-glutamyltransferase 2 (TGM2)
|
OT6MFOWF
|
TGM2_HUMAN
|
Affects Response To Substance
|
[9] |
|
G-protein-signaling modulator 2 (GPSM2)
|
OT6RPMRM
|
GPSM2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Cytochrome b5 (CYB5A)
|
OT6SN4RM
|
CYB5_HUMAN
|
Affects Response To Substance
|
[9] |
|
Ankycorbin (RAI14)
|
OT6USGBK
|
RAI14_HUMAN
|
Affects Response To Substance
|
[9] |
|
Plasminogen activator inhibitor 2 (SERPINB2)
|
OT72QLZB
|
PAI2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Replication protein A 70 kDa DNA-binding subunit (RPA1)
|
OT76POLP
|
RFA1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Signal transducer CD24 (CD24)
|
OT7CODJ4
|
CD24_HUMAN
|
Affects Response To Substance
|
[9] |
|
Polypeptide N-acetylgalactosaminyltransferase 3 (GALNT3)
|
OT7M67WT
|
GALT3_HUMAN
|
Affects Response To Substance
|
[9] |
|
Fibroblast growth factor 2 (FGF2)
|
OT7YUJ9F
|
FGF2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Forkhead box protein D1 (FOXD1)
|
OT80PRHS
|
FOXD1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Cbp/p300-interacting transactivator 2 (CITED2)
|
OT812TV7
|
CITE2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Raftlin (RFTN1)
|
OT8875JE
|
RFTN1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Mesoderm-specific transcript homolog protein (MEST)
|
OT8Q4U8Y
|
MEST_HUMAN
|
Affects Response To Substance
|
[9] |
|
Four-jointed box protein 1 (FJX1)
|
OT8SVTSS
|
FJX1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Leptin receptor (LEPR)
|
OT9H7G0C
|
LEPR_HUMAN
|
Affects Response To Substance
|
[9] |
|
Calmodulin-regulated spectrin-associated protein 2 (CAMSAP2)
|
OT9KXWE7
|
CAMP2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Oxidoreductase HTATIP2 (HTATIP2)
|
OT9MZ4QO
|
HTAI2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Tissue factor pathway inhibitor (TFPI)
|
OTA0FX16
|
TFPI1_HUMAN
|
Affects Response To Substance
|
[9] |
|
High mobility group nucleosome-binding domain-containing protein 3 (HMGN3)
|
OTA2TBWS
|
HMGN3_HUMAN
|
Affects Response To Substance
|
[9] |
|
Diphosphoinositol polyphosphate phosphohydrolase NUDT4B (NUDT4)
|
OTA6ICI2
|
NUD4B_HUMAN
|
Affects Response To Substance
|
[9] |
|
SEC14-like protein 1 (SEC14L1)
|
OTA75FET
|
S14L1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Autism susceptibility gene 2 protein (AUTS2)
|
OTAEXHSC
|
AUTS2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Grancalcin (GCA)
|
OTAJ7ZHG
|
GRAN_HUMAN
|
Affects Response To Substance
|
[9] |
|
RNA polymerase II elongation factor ELL3 (ELL3)
|
OTALC1YD
|
ELL3_HUMAN
|
Affects Response To Substance
|
[9] |
|
Receptor-type tyrosine-protein phosphatase kappa (PTPRK)
|
OTAP5AT3
|
PTPRK_HUMAN
|
Affects Response To Substance
|
[9] |
|
snRNA-activating protein complex subunit 1 (SNAPC1)
|
OTAPN1NI
|
SNPC1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Cyclin-dependent kinase inhibitor 1C (CDKN1C)
|
OTASTJ3Q
|
CDN1C_HUMAN
|
Affects Response To Substance
|
[9] |
|
Stearoyl-CoA desaturase (SCD)
|
OTB1073G
|
SCD_HUMAN
|
Affects Response To Substance
|
[9] |
|
Desmoplakin (DSP)
|
OTB2MOP8
|
DESP_HUMAN
|
Affects Response To Substance
|
[9] |
|
Protein lin-7 homolog C (LIN7C)
|
OTB3015O
|
LIN7C_HUMAN
|
Affects Response To Substance
|
[9] |
|
Heterogeneous nuclear ribonucleoprotein D-like (HNRNPDL)
|
OTB3BFCV
|
HNRDL_HUMAN
|
Affects Response To Substance
|
[9] |
|
High mobility group protein HMGI-C (HMGA2)
|
OTBMBHHL
|
HMGA2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Microtubule-associated tumor suppressor 1 (MTUS1)
|
OTBPALMU
|
MTUS1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Cilium assembly protein DZIP1 (DZIP1)
|
OTBVPO66
|
DZIP1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Thiamin pyrophosphokinase 1 (TPK1)
|
OTCHPUD0
|
TPK1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Cytoskeleton-associated protein 2 (CKAP2)
|
OTCLTC0J
|
CKAP2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Hydroxymethylglutaryl-CoA synthase, cytoplasmic (HMGCS1)
|
OTCO26FV
|
HMCS1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Cytochrome c oxidase copper chaperone (COX17)
|
OTCP4LH2
|
COX17_HUMAN
|
Affects Response To Substance
|
[9] |
|
Tetraspanin-13 (TSPAN13)
|
OTCS9BZY
|
TSN13_HUMAN
|
Affects Response To Substance
|
[9] |
|
S-phase kinase-associated protein 2 (SKP2)
|
OTD5B41X
|
SKP2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Tropomyosin alpha-1 chain (TPM1)
|
OTD73X6R
|
TPM1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Keratin, type I cytoskeletal 19 (KRT19)
|
OTDGDQ75
|
K1C19_HUMAN
|
Affects Response To Substance
|
[9] |
|
Integrin alpha-1 (ITGA1)
|
OTDITIST
|
ITA1_HUMAN
|
Increases Response To Substance
|
[10] |
|
AP-5 complex subunit mu-1 (AP5M1)
|
OTDLWEBL
|
AP5M1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Protein eva-1 homolog B (EVA1B)
|
OTDNAKQD
|
EVA1B_HUMAN
|
Affects Response To Substance
|
[9] |
|
Gamma-adducin (ADD3)
|
OTDRSHAZ
|
ADDG_HUMAN
|
Affects Response To Substance
|
[9] |
|
Dickkopf-related protein 3 (DKK3)
|
OTDU94EY
|
DKK3_HUMAN
|
Affects Response To Substance
|
[9] |
|
Ras-related protein Rab-7L1 (RAB29)
|
OTDZT6LP
|
RAB7L_HUMAN
|
Affects Response To Substance
|
[9] |
|
Short transient receptor potential channel 1 (TRPC1)
|
OTEEAGLH
|
TRPC1_HUMAN
|
Affects Response To Substance
|
[9] |
|
H(+)/Cl(-) exchange transporter 3 (CLCN3)
|
OTEEXNMU
|
CLCN3_HUMAN
|
Affects Response To Substance
|
[9] |
|
Secernin-1 (SCRN1)
|
OTELM5C2
|
SCRN1_HUMAN
|
Affects Response To Substance
|
[9] |
|
NGFI-A-binding protein 1 (NAB1)
|
OTEWHIZR
|
NAB1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Serglycin (SRGN)
|
OTFAVSX0
|
SRGN_HUMAN
|
Affects Response To Substance
|
[9] |
|
Solute carrier family 35 member G2 (SLC35G2)
|
OTFBVS0P
|
S35G2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Cadherin-1 (CDH1)
|
OTFJMXPM
|
CADH1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Multiple C2 and transmembrane domain-containing protein 2 (MCTP2)
|
OTFMZ8I2
|
MCTP2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Caveolae-associated protein 1 (CAVIN1)
|
OTFO915U
|
CAVN1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Laminin subunit alpha-2 (LAMA2)
|
OTFROQWE
|
LAMA2_HUMAN
|
Increases Response To Substance
|
[10] |
|
Centriolar satellite-associated tubulin polyglutamylase complex regulator 1 (CSTPP1)
|
OTFSO3XV
|
CSTP1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Baculoviral IAP repeat-containing protein 2 (BIRC2)
|
OTFXFREP
|
BIRC2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Myelin basic protein (MBP)
|
OTFZCEDB
|
MBP_HUMAN
|
Affects Response To Substance
|
[9] |
|
Transmembrane protein 80 (TMEM80)
|
OTG9D4KX
|
TMM80_HUMAN
|
Affects Response To Substance
|
[9] |
|
Squalene synthase (FDFT1)
|
OTGDISIT
|
FDFT_HUMAN
|
Affects Response To Substance
|
[9] |
|
High mobility group protein B2 (HMGB2)
|
OTGEGAOK
|
HMGB2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Cytotoxic granule associated RNA binding protein TIA1 (TIA1)
|
OTGPN3P8
|
TIA1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Stanniocalcin-1 (STC1)
|
OTGVVXYF
|
STC1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Sigma intracellular receptor 2 (TMEM97)
|
OTH76ZWK
|
SGMR2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Epithelial cell adhesion molecule (EPCAM)
|
OTHBZK5X
|
EPCAM_HUMAN
|
Affects Response To Substance
|
[9] |
|
Delta-aminolevulinic acid dehydratase (ALAD)
|
OTHM9GSH
|
HEM2_HUMAN
|
Affects Response To Substance
|
[9] |
|
DNA damage-inducible transcript 4 protein (DDIT4)
|
OTHY8SY4
|
DDIT4_HUMAN
|
Affects Response To Substance
|
[9] |
|
Interstitial collagenase (MMP1)
|
OTI4I2V1
|
MMP1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Solute carrier family 38 member 6 (SLC38A6)
|
OTIC8SHQ
|
S38A6_HUMAN
|
Affects Response To Substance
|
[9] |
|
Histone-lysine N-methyltransferase EZH2 (EZH2)
|
OTIEHTKW
|
EZH2_HUMAN
|
Affects Response To Substance
|
[9] |
|
G2/mitotic-specific cyclin-B2 (CCNB2)
|
OTIEXTDK
|
CCNB2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Laminin subunit gamma-1 (LAMC1)
|
OTIG527N
|
LAMC1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Cyclin-G2 (CCNG2)
|
OTII38K2
|
CCNG2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Beta/gamma crystallin domain-containing protein 1 (CRYBG1)
|
OTIPDI15
|
CRBG1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Ras-related protein Rab-13 (RAB13)
|
OTIQ484I
|
RAB13_HUMAN
|
Affects Response To Substance
|
[9] |
|
Insulin-like growth factor-binding protein 3 (IGFBP3)
|
OTIX63TX
|
IBP3_HUMAN
|
Affects Response To Substance
|
[9] |
|
Ankyrin repeat domain-containing protein 10 (ANKRD10)
|
OTIZ2VK8
|
ANR10_HUMAN
|
Affects Response To Substance
|
[9] |
|
Histone deacetylase complex subunit SAP18 (SAP18)
|
OTJAZWOL
|
SAP18_HUMAN
|
Affects Response To Substance
|
[9] |
|
E3 ubiquitin-protein ligase FANCL (FANCL)
|
OTJC7QPQ
|
FANCL_HUMAN
|
Affects Response To Substance
|
[9] |
|
Hydroxyacyl-coenzyme A dehydrogenase, mitochondrial (HADH)
|
OTJDOL20
|
HCDH_HUMAN
|
Affects Response To Substance
|
[9] |
|
FERM domain-containing protein 4A (FRMD4A)
|
OTJDTIK2
|
FRM4A_HUMAN
|
Affects Response To Substance
|
[9] |
|
Cyclin-F (CCNF)
|
OTJFVU43
|
CCNF_HUMAN
|
Affects Response To Substance
|
[9] |
|
Osteopontin (SPP1)
|
OTJGC23Y
|
OSTP_HUMAN
|
Affects Response To Substance
|
[9] |
|
Jupiter microtubule associated homolog 2 (JPT2)
|
OTJGPA4T
|
JUPI2_HUMAN
|
Affects Response To Substance
|
[9] |
|
TBC1 domain family member 4 (TBC1D4)
|
OTK66C40
|
TBCD4_HUMAN
|
Affects Response To Substance
|
[9] |
|
Tumor necrosis factor receptor superfamily member 6B (TNFRSF6B)
|
OTKAN9G7
|
TNF6B_HUMAN
|
Affects Response To Substance
|
[9] |
|
Myelin protein zero-like protein 2 (MPZL2)
|
OTKFNDUI
|
MPZL2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Transmembrane channel-like protein 6 (TMC6)
|
OTKH50J4
|
TMC6_HUMAN
|
Affects Response To Substance
|
[9] |
|
Procollagen-lysine,2-oxoglutarate 5-dioxygenase 2 (PLOD2)
|
OTKOZRZP
|
PLOD2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Methylsterol monooxygenase 1 (MSMO1)
|
OTKV62RZ
|
MSMO1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Enolase-phosphatase E1 (ENOPH1)
|
OTKXMWNN
|
ENOPH_HUMAN
|
Affects Response To Substance
|
[9] |
|
Calcium-binding tyrosine phosphorylation-regulated protein (CABYR)
|
OTKYMT99
|
CABYR_HUMAN
|
Affects Response To Substance
|
[9] |
|
Glycylpeptide N-tetradecanoyltransferase 2 (NMT2)
|
OTLNA39Z
|
NMT2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Hemicentin-1 (HMCN1)
|
OTLZAS0P
|
HMCN1_HUMAN
|
Increases Response To Substance
|
[10] |
|
Ribosome biogenesis protein BOP1 (BOP1)
|
OTMZMQDO
|
BOP1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Eukaryotic peptide chain release factor GTP-binding subunit ERF3B (GSPT2)
|
OTN0K7F3
|
ERF3B_HUMAN
|
Affects Response To Substance
|
[9] |
|
Four and a half LIM domains protein 1 (FHL1)
|
OTN535SU
|
FHL1_HUMAN
|
Affects Response To Substance
|
[9] |
|
MAP kinase-interacting serine/threonine-protein kinase 2 (MKNK2)
|
OTNA9G8V
|
MKNK2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Epithelial splicing regulatory protein 1 (ESRP1)
|
OTNCS4SL
|
ESRP1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Radixin (RDX)
|
OTNSYUN6
|
RADI_HUMAN
|
Affects Response To Substance
|
[9] |
|
Calcium-activated potassium channel subunit alpha-1 (KCNMA1)
|
OTNYXOZO
|
KCMA1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Paraneoplastic antigen-like protein 8A (PNMA8A)
|
OTO3F7B0
|
PNM8A_HUMAN
|
Affects Response To Substance
|
[9] |
|
Polypeptide N-acetylgalactosaminyltransferase 1 (GALNT1)
|
OTO3RO36
|
GALT1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Histone deacetylase 9 (HDAC9)
|
OTO8O0LF
|
HDAC9_HUMAN
|
Affects Response To Substance
|
[9] |
|
Histone-lysine N-methyltransferase KMT5B (KMT5B)
|
OTOH7G8Q
|
KMT5B_HUMAN
|
Affects Response To Substance
|
[9] |
|
BH3-interacting domain death agonist (BID)
|
OTOSHSHU
|
BID_HUMAN
|
Affects Response To Substance
|
[9] |
|
GTP cyclohydrolase 1 (GCH1)
|
OTOZ6NSL
|
GCH1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Unconventional myosin-Id (MYO1D)
|
OTP2RGPN
|
MYO1D_HUMAN
|
Affects Response To Substance
|
[9] |
|
Rho GTPase-activating protein 7 (DLC1)
|
OTP8LMCR
|
RHG07_HUMAN
|
Affects Response To Substance
|
[9] |
|
Ras-related protein Rab-20 (RAB20)
|
OTP9OOVS
|
RAB20_HUMAN
|
Affects Response To Substance
|
[9] |
|
Dual specificity protein phosphatase 3 (DUSP3)
|
OTPJX9B4
|
DUS3_HUMAN
|
Affects Response To Substance
|
[9] |
|
DNA dC->dU-editing enzyme APOBEC-3C (APOBEC3C)
|
OTPL0AI1
|
ABC3C_HUMAN
|
Affects Response To Substance
|
[9] |
|
Pentraxin-related protein PTX3 (PTX3)
|
OTPXHRKU
|
PTX3_HUMAN
|
Affects Response To Substance
|
[9] |
|
Dermatan-sulfate epimerase (DSE)
|
OTQ108VJ
|
DSE_HUMAN
|
Affects Response To Substance
|
[9] |
|
Centromere protein U (CENPU)
|
OTQ4TZRS
|
CENPU_HUMAN
|
Affects Response To Substance
|
[9] |
|
Eukaryotic translation initiation factor 5A-1 (EIF5A)
|
OTQ8DJX5
|
IF5A1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Sortilin-related receptor (SORL1)
|
OTQ8FFNS
|
SORL_HUMAN
|
Affects Response To Substance
|
[9] |
|
Collagen alpha-2(VI) chain (COL6A2)
|
OTQC6PPO
|
CO6A2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Ran-specific GTPase-activating protein (RANBP1)
|
OTQE226K
|
RANG_HUMAN
|
Affects Response To Substance
|
[9] |
|
MAPK/MAK/MRK overlapping kinase (MOK)
|
OTQK7M9V
|
MOK_HUMAN
|
Affects Response To Substance
|
[9] |
|
3-oxo-5-alpha-steroid 4-dehydrogenase 1 (SRD5A1)
|
OTQRET2B
|
S5A1_HUMAN
|
Affects Response To Substance
|
[9] |
|
High mobility group protein HMG-I/HMG-Y (HMGA1)
|
OTQUSHPX
|
HMGA1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Keratin, type I cytoskeletal 23 (KRT23)
|
OTQW6UAG
|
K1C23_HUMAN
|
Increases Response To Substance
|
[10] |
|
Transforming growth factor-beta-induced protein ig-h3 (TGFBI)
|
OTR443C5
|
BGH3_HUMAN
|
Decreases Response To Substance
|
[10] |
|
Large ribosomal subunit protein mL63 (MRPL57)
|
OTRB659E
|
RT63_HUMAN
|
Affects Response To Substance
|
[9] |
|
Actin, gamma-enteric smooth muscle (ACTG2)
|
OTRDWUO0
|
ACTH_HUMAN
|
Affects Response To Substance
|
[9] |
|
Acidic leucine-rich nuclear phosphoprotein 32 family member A (ANP32A)
|
OTRHPFO2
|
AN32A_HUMAN
|
Affects Response To Substance
|
[9] |
|
LIM domain kinase 2 (LIMK2)
|
OTRJD7VZ
|
LIMK2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Calcium/calmodulin-dependent protein kinase type 1 (CAMK1)
|
OTRN55RE
|
KCC1A_HUMAN
|
Affects Response To Substance
|
[9] |
|
Receptor tyrosine-protein kinase erbB-3 (ERBB3)
|
OTRSST0A
|
ERBB3_HUMAN
|
Affects Response To Substance
|
[9] |
|
Lysyl oxidase homolog 2 (LOXL2)
|
OTRT46B5
|
LOXL2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Arginine and glutamate-rich protein 1 (ARGLU1)
|
OTRVRZ4Z
|
ARGL1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Dr1-associated corepressor (DRAP1)
|
OTRWLF6D
|
NC2A_HUMAN
|
Affects Response To Substance
|
[9] |
|
Latent-transforming growth factor beta-binding protein 2 (LTBP2)
|
OTS88GSD
|
LTBP2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Heat shock-related 70 kDa protein 2 (HSPA2)
|
OTSDET7B
|
HSP72_HUMAN
|
Affects Response To Substance
|
[9] |
|
Inhibin beta A chain (INHBA)
|
OTSP64PQ
|
INHBA_HUMAN
|
Affects Response To Substance
|
[9] |
|
Lumican (LUM)
|
OTSRC874
|
LUM_HUMAN
|
Decreases Response To Substance
|
[10] |
|
GTP-binding protein SAR1a (SAR1A)
|
OTSSRVGV
|
SAR1A_HUMAN
|
Affects Response To Substance
|
[9] |
|
Plasminogen activator inhibitor 1 (SERPINE1)
|
OTT0MPQ3
|
PAI1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Collagen alpha-1(III) chain (COL3A1)
|
OTT1EMLM
|
CO3A1_HUMAN
|
Decreases Response To Substance
|
[10] |
|
DNA-binding protein SATB1 (SATB1)
|
OTT7SUVW
|
SATB1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Gap junction alpha-1 protein (GJA1)
|
OTT94MKL
|
CXA1_HUMAN
|
Affects Response To Substance
|
[9] |
|
KH domain-containing RNA-binding protein QKI (QKI)
|
OTTAUGLB
|
QKI_HUMAN
|
Affects Response To Substance
|
[9] |
|
Cullin-4A (CUL4A)
|
OTTBV70J
|
CUL4A_HUMAN
|
Affects Response To Substance
|
[9] |
|
Homeobox protein Hox-B2 (HOXB2)
|
OTTD6HMV
|
HXB2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Collagen alpha-1(XV) chain (COL15A1)
|
OTTFKK18
|
COFA1_HUMAN
|
Decreases Response To Substance
|
[10] |
|
A disintegrin and metalloproteinase with thrombospondin motifs 1 (ADAMTS1)
|
OTTLJH8W
|
ATS1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Nuclear receptor-interacting protein 3 (NRIP3)
|
OTU15GMK
|
NRIP3_HUMAN
|
Affects Response To Substance
|
[9] |
|
Granulocyte-macrophage colony-stimulating factor receptor subunit alpha (CSF2RA)
|
OTUOO2XS
|
CSF2R_HUMAN
|
Affects Response To Substance
|
[9] |
|
La-related protein 6 (LARP6)
|
OTUQ9QS9
|
LARP6_HUMAN
|
Affects Response To Substance
|
[9] |
|
General transcription factor II-I (GTF2I)
|
OTUYL1TQ
|
GTF2I_HUMAN
|
Affects Response To Substance
|
[9] |
|
Protein aurora borealis (BORA)
|
OTV2ZPKP
|
BORA_HUMAN
|
Affects Response To Substance
|
[9] |
|
FYVE, RhoGEF and PH domain-containing protein 1 (FGD1)
|
OTV3T64P
|
FGD1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Matrilin-2 (MATN2)
|
OTVDR68G
|
MATN2_HUMAN
|
Decreases Response To Substance
|
[10] |
|
Microtubule-actin cross-linking factor 1, isoforms 1/2/3/4/5 (MACF1)
|
OTVIHD77
|
MACF1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Serine protease FAM111A (FAM111A)
|
OTVLARLG
|
F111A_HUMAN
|
Affects Response To Substance
|
[9] |
|
Transcription factor 7-like 2 (TCF7L2)
|
OTVWPZ8B
|
TF7L2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Interleukin-1 receptor accessory protein-like 1 (IL1RAPL1)
|
OTW3T4B2
|
IRPL1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Transcription factor E2-alpha (TCF3)
|
OTW6FR32
|
TFE2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Tissue alpha-L-fucosidase (FUCA1)
|
OTW71IK4
|
FUCO_HUMAN
|
Affects Response To Substance
|
[9] |
|
Fanconi anemia group I protein (FANCI)
|
OTW8E3SC
|
FANCI_HUMAN
|
Affects Response To Substance
|
[9] |
|
U2 small nuclear ribonucleoprotein A' (SNRPA1)
|
OTWJZFAP
|
RU2A_HUMAN
|
Affects Response To Substance
|
[9] |
|
Decorin (DCN)
|
OTWLJL7K
|
PGS2_HUMAN
|
Decreases Response To Substance
|
[10] |
|
Periostin (POSTN)
|
OTWP62UA
|
POSTN_HUMAN
|
Decreases Response To Substance
|
[10] |
|
Methyl-CpG-binding domain protein 4 (MBD4)
|
OTWR9YXE
|
MBD4_HUMAN
|
Affects Response To Substance
|
[9] |
|
Glypican-3 (GPC3)
|
OTWS8WSW
|
GPC3_HUMAN
|
Increases Response To Substance
|
[10] |
|
GDP-mannose 4,6 dehydratase (GMDS)
|
OTWV79YD
|
GMDS_HUMAN
|
Affects Response To Substance
|
[9] |
|
ORC ubiquitin ligase 1 (OBI1)
|
OTX62ZHW
|
OBI1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Caspase-6 (CASP6)
|
OTXLD3EC
|
CASP6_HUMAN
|
Affects Response To Substance
|
[9] |
|
Condensin complex subunit 2 (NCAPH)
|
OTXOS97C
|
CND2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Collagen alpha-2(I) chain (COL1A2)
|
OTY7G382
|
CO1A2_HUMAN
|
Increases Response To Substance
|
[10] |
|
Collagen alpha-1(VI) chain (COL6A1)
|
OTYKSCOB
|
CO6A1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Protein FAN (NSMAF)
|
OTYNVZ23
|
FAN_HUMAN
|
Affects Response To Substance
|
[9] |
|
Protein Niban 1 (NIBAN1)
|
OTYOLI12
|
NIBA1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Nucleolar complex protein 3 homolog (NOC3L)
|
OTYOR3PV
|
NOC3L_HUMAN
|
Affects Response To Substance
|
[9] |
|
Procathepsin L (CTSL)
|
OTYTUW29
|
CATL1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Acetyl-CoA acetyltransferase, cytosolic (ACAT2)
|
OTZ092ZJ
|
THIC_HUMAN
|
Affects Response To Substance
|
[9] |
|
Thiosulfate sulfurtransferase (TST)
|
OTZ4F5LK
|
THTR_HUMAN
|
Affects Response To Substance
|
[9] |
|
Tissue factor pathway inhibitor 2 (TFPI2)
|
OTZCRWOR
|
TFPI2_HUMAN
|
Affects Response To Substance
|
[9] |
|
Insulin-induced gene 1 protein (INSIG1)
|
OTZF5X1D
|
INSI1_HUMAN
|
Affects Response To Substance
|
[9] |
|
Suppressor of tumorigenicity 7 protein (ST7)
|
OTZG8RC6
|
ST7_HUMAN
|
Affects Response To Substance
|
[9] |
|
Fermitin family homolog 2 (FERMT2)
|
OTZNPWWX
|
FERM2_HUMAN
|
Affects Response To Substance
|
[9] |
| ------------------------------------------------------------------------------------ |
|
|
|
|