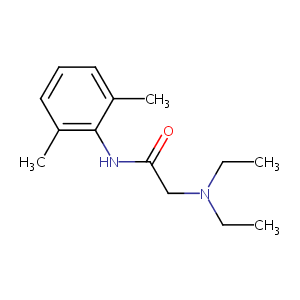
| DOT Name |
DOT ID |
UniProt ID |
Mode of Action |
REF |
|
Cytochrome P450 3A4 (CYP3A4)
|
OTQGYY83
|
CP3A4_HUMAN
|
Decreases Activity
|
[15] |
|
Cytochrome P450 2C9 (CYP2C9)
|
OTGLBN29
|
CP2C9_HUMAN
|
Decreases Activity
|
[16] |
|
Cytochrome P450 2D6 (CYP2D6)
|
OTZJC802
|
CP2D6_HUMAN
|
Decreases Activity
|
[16] |
|
Cytochrome P450 2C19 (CYP2C19)
|
OTFMJYYE
|
CP2CJ_HUMAN
|
Decreases Activity
|
[16] |
|
Cytochrome P450 1A2 (CYP1A2)
|
OTLLBX48
|
CP1A2_HUMAN
|
Decreases Activity
|
[16] |
|
Heme oxygenase 1 (HMOX1)
|
OTC1W6UX
|
HMOX1_HUMAN
|
Increases Expression
|
[17] |
|
RAC-alpha serine/threonine-protein kinase (AKT1)
|
OT8H2YY7
|
AKT1_HUMAN
|
Increases Phosphorylation
|
[17] |
|
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
|
OT0HENJ5
|
NF2L2_HUMAN
|
Increases Localization
|
[17] |
|
NPC intracellular cholesterol transporter 1 (NPC1)
|
OTRIPICX
|
NPC1_HUMAN
|
Increases Expression
|
[18] |
|
Delta(14)-sterol reductase TM7SF2 (TM7SF2)
|
OTILU5S7
|
ERG24_HUMAN
|
Decreases Expression
|
[18] |
|
Fatty acid CoA ligase Acsl3 (ACSL3)
|
OT3MWER1
|
ACSL3_HUMAN
|
Decreases Expression
|
[18] |
|
Transforming growth factor beta-1 proprotein (TGFB1)
|
OTV5XHVH
|
TGFB1_HUMAN
|
Decreases Expression
|
[18] |
|
Apolipoprotein C-I (APOC1)
|
OTA58CED
|
APOC1_HUMAN
|
Decreases Expression
|
[18] |
|
Fatty acid-binding protein, adipocyte (FABP4)
|
OT3DKFOU
|
FABP4_HUMAN
|
Decreases Expression
|
[18] |
|
Cyclic AMP-dependent transcription factor ATF-4 (ATF4)
|
OTRFV19J
|
ATF4_HUMAN
|
Increases Expression
|
[18] |
|
Sterol carrier protein 2 (SCP2)
|
OTPAFCPQ
|
SCP2_HUMAN
|
Decreases Expression
|
[18] |
|
Interleukin-12 subunit beta (IL12B)
|
OT0JF8A3
|
IL12B_HUMAN
|
Decreases Expression
|
[18] |
|
Cyclin-dependent kinase inhibitor 2A (CDKN2A)
|
OTN0ZWAE
|
CDN2A_HUMAN
|
Increases Expression
|
[18] |
|
Hepatocyte nuclear factor 3-alpha (FOXA1)
|
OTEBY0TD
|
FOXA1_HUMAN
|
Decreases Expression
|
[18] |
|
Fatty acid-binding protein 5 (FABP5)
|
OTNM186C
|
FABP5_HUMAN
|
Decreases Expression
|
[18] |
|
Peroxisomal acyl-coenzyme A oxidase 1 (ACOX1)
|
OTM0A0DY
|
ACOX1_HUMAN
|
Increases Expression
|
[18] |
|
Methylsterol monooxygenase 1 (MSMO1)
|
OTKV62RZ
|
MSMO1_HUMAN
|
Increases Expression
|
[18] |
|
Eukaryotic translation initiation factor 2A (EIF2A)
|
OTWXELQP
|
EIF2A_HUMAN
|
Increases Phosphorylation
|
[18] |
|
Phosphatidylcholine transfer protein (PCTP)
|
OTM36JXE
|
PPCT_HUMAN
|
Decreases Expression
|
[18] |
|
Nitric oxide synthase 3 (NOS3)
|
OTLDT7NR
|
NOS3_HUMAN
|
Increases Expression
|
[19] |
|
Nitric oxide synthase 1 (NOS1)
|
OT7M8XVG
|
NOS1_HUMAN
|
Increases Expression
|
[19] |
|
Nitric oxide synthase, inducible (NOS2)
|
OTKKIOJ1
|
NOS2_HUMAN
|
Increases Expression
|
[19] |
|
Phosphoglucomutase-1 (PGM1)
|
OT3VM4JX
|
PGM1_HUMAN
|
Increases Expression
|
[20] |
|
Triosephosphate isomerase (TPI1)
|
OT14KP4B
|
TPIS_HUMAN
|
Increases Expression
|
[20] |
|
Tumor necrosis factor receptor superfamily member 10A (TNFRSF10A)
|
OTBPCU2O
|
TR10A_HUMAN
|
Increases Expression
|
[21] |
|
DNA fragmentation factor subunit alpha (DFFA)
|
OTFPFMT8
|
DFFA_HUMAN
|
Decreases Expression
|
[22] |
|
Apoptotic protease-activating factor 1 (APAF1)
|
OTJWIVY0
|
APAF_HUMAN
|
Increases Expression
|
[21] |
|
Tumor necrosis factor receptor superfamily member 10B (TNFRSF10B)
|
OTA1CPBV
|
TR10B_HUMAN
|
Increases Expression
|
[21] |
|
Angiopoietin-2 (ANGPT2)
|
OTEQK65P
|
ANGP2_HUMAN
|
Increases Expression
|
[23] |
|
Baculoviral IAP repeat-containing protein 5 (BIRC5)
|
OTILXZYL
|
BIRC5_HUMAN
|
Decreases Expression
|
[24] |
|
CASP8 and FADD-like apoptosis regulator (CFLAR)
|
OTX14BAS
|
CFLAR_HUMAN
|
Increases Expression
|
[21] |
|
Receptor-interacting serine/threonine-protein kinase 2 (RIPK2)
|
OT0KMIYQ
|
RIPK2_HUMAN
|
Increases Expression
|
[21] |
|
BCL2/adenovirus E1B 19 kDa protein-interacting protein 3-like (BNIP3L)
|
OTJKOMXE
|
BNI3L_HUMAN
|
Decreases Expression
|
[21] |
|
Nuclear protein 1 (NUPR1)
|
OT4FU8C0
|
NUPR1_HUMAN
|
Increases Expression
|
[7] |
|
Serine/threonine-protein kinase/endoribonuclease IRE1 (ERN1)
|
OTY9R6FZ
|
ERN1_HUMAN
|
Increases Expression
|
[25] |
|
Transient receptor potential cation channel subfamily A member 1 (TRPA1)
|
OTRDIR5M
|
TRPA1_HUMAN
|
Increases Activity
|
[26] |
|
BAG family molecular chaperone regulator 3 (BAG3)
|
OTVXYUDQ
|
BAG3_HUMAN
|
Increases Expression
|
[21] |
|
Apoptosis-inducing factor 1, mitochondrial (AIFM1)
|
OTKPWB7Q
|
AIFM1_HUMAN
|
Affects Localization
|
[27] |
|
B-cell lymphoma/leukemia 10 (BCL10)
|
OT47MCLI
|
BCL10_HUMAN
|
Increases Expression
|
[21] |
|
Serine/threonine-protein kinase Chk2 (CHEK2)
|
OT8ZPCNS
|
CHK2_HUMAN
|
Increases Expression
|
[21] |
|
Glutamate dehydrogenase 1, mitochondrial (GLUD1)
|
OTXKOCUH
|
DHE3_HUMAN
|
Increases Expression
|
[28] |
|
Tyrosine-protein kinase ABL1 (ABL1)
|
OT09YVXH
|
ABL1_HUMAN
|
Increases Expression
|
[21] |
|
Epidermal growth factor receptor (EGFR)
|
OTAPLO1S
|
EGFR_HUMAN
|
Decreases Expression
|
[29] |
|
Urokinase-type plasminogen activator (PLAU)
|
OTX0QGKK
|
UROK_HUMAN
|
Increases Expression
|
[23] |
|
Metalloproteinase inhibitor 1 (TIMP1)
|
OTOXC51H
|
TIMP1_HUMAN
|
Increases Expression
|
[23] |
|
Protein c-Fos (FOS)
|
OTJBUVWS
|
FOS_HUMAN
|
Decreases Expression
|
[21] |
|
Myc proto-oncogene protein (MYC)
|
OTPV5LUK
|
MYC_HUMAN
|
Increases Expression
|
[21] |
|
GTPase HRas (HRAS)
|
OTWQN0DP
|
RASH_HUMAN
|
Increases Expression
|
[30] |
|
Calcitonin (CALCA)
|
OTZ11LHB
|
CALC_HUMAN
|
Increases Expression
|
[31] |
|
Tumor necrosis factor (TNF)
|
OT4IE164
|
TNFA_HUMAN
|
Increases Expression
|
[32] |
|
Albumin (ALB)
|
OTVMM513
|
ALBU_HUMAN
|
Increases Expression
|
[28] |
|
Lactotransferrin (LTF)
|
OT8JWCZ0
|
TRFL_HUMAN
|
Increases Expression
|
[33] |
|
Interstitial collagenase (MMP1)
|
OTI4I2V1
|
MMP1_HUMAN
|
Increases Expression
|
[23] |
|
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
|
OTBPMIMW
|
G3P_HUMAN
|
Increases Expression
|
[28] |
|
Receptor tyrosine-protein kinase erbB-2 (ERBB2)
|
OTOAUNCK
|
ERBB2_HUMAN
|
Decreases Expression
|
[29] |
|
Cellular tumor antigen p53 (TP53)
|
OTIE1VH3
|
P53_HUMAN
|
Increases Expression
|
[21] |
|
Insulin-like growth factor I (IGF1)
|
OTIIZR61
|
IGF1_HUMAN
|
Increases Expression
|
[23] |
|
Plasminogen activator inhibitor 1 (SERPINE1)
|
OTT0MPQ3
|
PAI1_HUMAN
|
Decreases Expression
|
[23] |
|
Eukaryotic translation initiation factor 2 subunit 1 (EIF2S1)
|
OTM0GDTP
|
IF2A_HUMAN
|
Increases Phosphorylation
|
[25] |
|
Interleukin-6 (IL6)
|
OTUOSCCU
|
IL6_HUMAN
|
Increases Expression
|
[34] |
|
Transcription factor Jun (JUN)
|
OTCYBO6X
|
JUN_HUMAN
|
Decreases Expression
|
[21] |
|
Transcription factor Sp1 (SP1)
|
OTISPT4X
|
SP1_HUMAN
|
Increases Localization
|
[35] |
|
Insulin-like growth factor 1 receptor (IGF1R)
|
OTXJIF13
|
IGF1R_HUMAN
|
Increases Expression
|
[23] |
|
ATP-dependent translocase ABCB1 (ABCB1)
|
OTEJROBO
|
MDR1_HUMAN
|
Increases Expression
|
[36] |
|
Galectin-1 (LGALS1)
|
OT8LDFWR
|
LEG1_HUMAN
|
Increases Expression
|
[28] |
|
Poly polymerase 1 (PARP1)
|
OT310QSG
|
PARP1_HUMAN
|
Increases Adp-Ribosylation
|
[37] |
|
Calcitonin gene-related peptide 2 (CALCB)
|
OTNVY7WY
|
CALCB_HUMAN
|
Increases Expression
|
[31] |
|
Interleukin-8 (CXCL8)
|
OTS7T5VH
|
IL8_HUMAN
|
Increases Expression
|
[34] |
|
Androgen receptor (AR)
|
OTUBKAZZ
|
ANDR_HUMAN
|
Increases Expression
|
[38] |
|
Apoptosis regulator Bcl-2 (BCL2)
|
OT9DVHC0
|
BCL2_HUMAN
|
Increases Expression
|
[21] |
|
Microtubule-associated protein tau (MAPT)
|
OTMTP2Z7
|
TAU_HUMAN
|
Increases Expression
|
[7] |
|
Endoplasmic reticulum chaperone BiP (HSPA5)
|
OTFUIRAO
|
BIP_HUMAN
|
Increases Expression
|
[25] |
|
Microtubule-associated protein 2 (MAP2)
|
OT6UYT3X
|
MTAP2_HUMAN
|
Increases Expression
|
[39] |
|
Granzyme A (GZMA)
|
OT43R33L
|
GRAA_HUMAN
|
Increases Expression
|
[23] |
|
Myosin light chain 4 (MYL4)
|
OTURFCSE
|
MYL4_HUMAN
|
Decreases Expression
|
[10] |
|
Cadherin-1 (CDH1)
|
OTFJMXPM
|
CADH1_HUMAN
|
Increases Expression
|
[7] |
|
Glial fibrillary acidic protein (GFAP)
|
OTQ01ZAS
|
GFAP_HUMAN
|
Increases Expression
|
[39] |
|
Endoplasmin (HSP90B1)
|
OT02XLBR
|
ENPL_HUMAN
|
Increases Expression
|
[28] |
|
G2/mitotic-specific cyclin-B1 (CCNB1)
|
OT19S7E5
|
CCNB1_HUMAN
|
Decreases Expression
|
[40] |
|
Matrix metalloproteinase-9 (MMP9)
|
OTB2QDAV
|
MMP9_HUMAN
|
Increases Expression
|
[23] |
|
NAD(P)H dehydrogenase 1 (NQO1)
|
OTZGGIVK
|
NQO1_HUMAN
|
Increases Expression
|
[10] |
|
Histone H2AX (H2AX)
|
OT18UX57
|
H2AX_HUMAN
|
Increases Localization
|
[37] |
|
Protein kinase C alpha type (PRKCA)
|
OT5UWNRD
|
KPCA_HUMAN
|
Increases Expression
|
[32] |
|
Cadherin-2 (CDH2)
|
OTH0Y56P
|
CADH2_HUMAN
|
Decreases Expression
|
[7] |
|
Tumor necrosis factor receptor superfamily member 1A (TNFRSF1A)
|
OT2D9DOV
|
TNR1A_HUMAN
|
Increases Expression
|
[23] |
|
Retinoic acid receptor RXR-alpha (RXRA)
|
OTP1TBDM
|
RXRA_HUMAN
|
Decreases Expression
|
[41] |
|
Nuclear factor NF-kappa-B p105 subunit (NFKB1)
|
OTNRRD8I
|
NFKB1_HUMAN
|
Affects Expression
|
[21] |
|
Cyclin-A2 (CCNA2)
|
OTPHHYZJ
|
CCNA2_HUMAN
|
Decreases Expression
|
[40] |
|
Protachykinin-1 (TAC1)
|
OTM842YW
|
TKN1_HUMAN
|
Decreases Expression
|
[42] |
|
Cytochrome c oxidase subunit 5A, mitochondrial (COX5A)
|
OTP0961M
|
COX5A_HUMAN
|
Increases Expression
|
[28] |
|
Nucleoside diphosphate kinase B (NME2)
|
OTCYGLHV
|
NDKB_HUMAN
|
Increases Expression
|
[23] |
|
Ribosomal protein S6 kinase beta-1 (RPS6KB1)
|
OTAELNGX
|
KS6B1_HUMAN
|
Increases Phosphorylation
|
[7] |
|
G1/S-specific cyclin-D1 (CCND1)
|
OT8HPTKJ
|
CCND1_HUMAN
|
Decreases Expression
|
[24] |
|
Growth arrest and DNA damage-inducible protein GADD45 alpha (GADD45A)
|
OTDRV63V
|
GA45A_HUMAN
|
Increases Expression
|
[21] |
|
Tenascin (TNC)
|
OTK4FSHR
|
TENA_HUMAN
|
Decreases Expression
|
[10] |
|
Cyclin-dependent kinase 2 (CDK2)
|
OTB5DYYZ
|
CDK2_HUMAN
|
Decreases Expression
|
[43] |
|
Tumor necrosis factor receptor superfamily member 6 (FAS)
|
OTP9XG86
|
TNR6_HUMAN
|
Increases Expression
|
[21] |
|
NF-kappa-B inhibitor alpha (NFKBIA)
|
OTFT924M
|
IKBA_HUMAN
|
Decreases Degradation
|
[44] |
|
Protein S100-A4 (S100A4)
|
OTLRGFSQ
|
S10A4_HUMAN
|
Increases Expression
|
[23] |
|
Mitogen-activated protein kinase 3 (MAPK3)
|
OTCYKGKO
|
MK03_HUMAN
|
Decreases Activity
|
[45] |
|
Mitogen-activated protein kinase 1 (MAPK1)
|
OTH85PI5
|
MK01_HUMAN
|
Decreases Activity
|
[45] |
|
Retinoic acid receptor RXR-beta (RXRB)
|
OTNPDXG2
|
RXRB_HUMAN
|
Decreases Expression
|
[41] |
|
Peroxiredoxin-6 (PRDX6)
|
OTS8KC8A
|
PRDX6_HUMAN
|
Increases Expression
|
[28] |
|
Metalloproteinase inhibitor 3 (TIMP3)
|
OTDGQAD1
|
TIMP3_HUMAN
|
Increases Expression
|
[23] |
|
DNA damage-inducible transcript 3 protein (DDIT3)
|
OTI8YKKE
|
DDIT3_HUMAN
|
Increases Expression
|
[46] |
|
Sterol regulatory element-binding protein 1 (SREBF1)
|
OTWBRPAI
|
SRBP1_HUMAN
|
Increases Expression
|
[7] |
|
Peroxisome proliferator-activated receptor gamma (PPARG)
|
OTHMARHO
|
PPARG_HUMAN
|
Decreases Expression
|
[41] |
|
Squalene synthase (FDFT1)
|
OTGDISIT
|
FDFT_HUMAN
|
Increases Expression
|
[10] |
|
Cyclin-dependent kinase inhibitor 1 (CDKN1A)
|
OTQWHCZE
|
CDN1A_HUMAN
|
Increases Expression
|
[21] |
|
Collagen alpha-1(XVIII) chain (COL18A1)
|
OTJFUH6O
|
COIA1_HUMAN
|
Increases Expression
|
[23] |
|
Signal transducer and activator of transcription 3 (STAT3)
|
OTAAGKYZ
|
STAT3_HUMAN
|
Affects Localization
|
[24] |
|
Serine/threonine-protein kinase mTOR (MTOR)
|
OTHH8KU7
|
MTOR_HUMAN
|
Decreases Phosphorylation
|
[37] |
|
Caspase-3 (CASP3)
|
OTIJRBE7
|
CASP3_HUMAN
|
Increases Activity
|
[47] |
|
Mitogen-activated protein kinase 8 (MAPK8)
|
OTEREYS5
|
MK08_HUMAN
|
Increases Activity
|
[45] |
|
Mitogen-activated protein kinase 9 (MAPK9)
|
OTCEVJ9E
|
MK09_HUMAN
|
Increases Activity
|
[48] |
|
5-hydroxytryptamine receptor 3A (HTR3A)
|
OTAIV1AK
|
5HT3A_HUMAN
|
Decreases Activity
|
[49] |
|
Cyclin-dependent kinase inhibitor 1B (CDKN1B)
|
OTNY5LLZ
|
CDN1B_HUMAN
|
Decreases Expression
|
[37] |
|
Cytosolic phospholipase A2 (PLA2G4A)
|
OTE70SOT
|
PA24A_HUMAN
|
Increases Phosphorylation
|
[50] |
|
Caspase-4 (CASP4)
|
OTVQTV1L
|
CASP4_HUMAN
|
Increases Activity
|
[51] |
|
Carnitine O-palmitoyltransferase 1, liver isoform (CPT1A)
|
OTI862QH
|
CPT1A_HUMAN
|
Increases Expression
|
[52] |
|
Tumor necrosis factor ligand superfamily member 10 (TNFSF10)
|
OT4PXBTA
|
TNF10_HUMAN
|
Increases Expression
|
[35] |
|
Death-associated protein 1 (DAP)
|
OT5YLL7E
|
DAP1_HUMAN
|
Increases Expression
|
[7] |
|
Ribosomal protein S6 kinase alpha-3 (RPS6KA3)
|
OTYJNNMD
|
KS6A3_HUMAN
|
Increases Expression
|
[28] |
|
Serine/threonine-protein kinase Nek2 (NEK2)
|
OT1H27HO
|
NEK2_HUMAN
|
Increases Expression
|
[10] |
|
5'-AMP-activated protein kinase catalytic subunit alpha-2 (PRKAA2)
|
OTU1KZPV
|
AAPK2_HUMAN
|
Increases Phosphorylation
|
[7] |
|
Caspase-7 (CASP7)
|
OTAPJ040
|
CASP7_HUMAN
|
Increases Activity
|
[25] |
|
Caspase-9 (CASP9)
|
OTD4RFFG
|
CASP9_HUMAN
|
Increases Expression
|
[21] |
|
BH3-interacting domain death agonist (BID)
|
OTOSHSHU
|
BID_HUMAN
|
Increases Expression
|
[21] |
|
Sestrin-3 (SESN3)
|
OTJRY1Y5
|
SESN3_HUMAN
|
Increases Expression
|
[7] |
|
Casein kinase II subunit beta (CSNK2B)
|
OT2WW7R1
|
CSK2B_HUMAN
|
Increases Phosphorylation
|
[53] |
|
Casein kinase II subunit alpha (CSNK2A1)
|
OT9T8WQM
|
CSK21_HUMAN
|
Increases Phosphorylation
|
[53] |
|
DNA-dependent protein kinase catalytic subunit (PRKDC)
|
OTJVS4EG
|
PRKDC_HUMAN
|
Increases Phosphorylation
|
[37] |
|
Mucin-5AC (MUC5AC)
|
OTJV8O04
|
MUC5A_HUMAN
|
Increases Secretion
|
[54] |
|
E3 ubiquitin-protein ligase Mdm2 (MDM2)
|
OTOVXARF
|
MDM2_HUMAN
|
Increases Expression
|
[21] |
|
Transcription factor E2F1 (E2F1)
|
OTLKYBBC
|
E2F1_HUMAN
|
Increases Expression
|
[21] |
|
Dual specificity mitogen-activated protein kinase kinase 1 (MAP2K1)
|
OT4Y9NQI
|
MP2K1_HUMAN
|
Increases Expression
|
[21] |
|
Angiopoietin-1 receptor (TEK)
|
OT78YN57
|
TIE2_HUMAN
|
Increases Expression
|
[23] |
|
Peroxisome proliferator-activated receptor delta (PPARD)
|
OTI4WTOP
|
PPARD_HUMAN
|
Decreases Expression
|
[41] |
|
Urokinase plasminogen activator surface receptor (PLAUR)
|
OTIRKKEQ
|
UPAR_HUMAN
|
Increases Expression
|
[23] |
|
Transcription factor p65 (RELA)
|
OTUJP9CN
|
TF65_HUMAN
|
Decreases Localization
|
[44] |
|
Protein kinase C delta type (PRKCD)
|
OTSEH90E
|
KPCD_HUMAN
|
Increases Expression
|
[55] |
|
Apoptosis regulator BAX (BAX)
|
OTAW0V4V
|
BAX_HUMAN
|
Increases Expression
|
[56] |
|
Bcl-2-like protein 1 (BCL2L1)
|
OTRC5K9O
|
B2CL1_HUMAN
|
Increases Expression
|
[21] |
|
Induced myeloid leukemia cell differentiation protein Mcl-1 (MCL1)
|
OT2YYI1A
|
MCL1_HUMAN
|
Increases Expression
|
[21] |
|
Peroxisome proliferator-activated receptor alpha (PPARA)
|
OTK095PP
|
PPARA_HUMAN
|
Decreases Expression
|
[41] |
|
BCL2/adenovirus E1B 19 kDa protein-interacting protein 2 (BNIP2)
|
OTVZD4H6
|
BNIP2_HUMAN
|
Increases Expression
|
[21] |
|
BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 (BNIP3)
|
OT4SO7J4
|
BNIP3_HUMAN
|
Decreases Expression
|
[21] |
|
TNF receptor-associated factor 3 (TRAF3)
|
OT5TQBGV
|
TRAF3_HUMAN
|
Increases Expression
|
[21] |
|
5'-AMP-activated protein kinase catalytic subunit alpha-1 (PRKAA1)
|
OT7TNF0L
|
AAPK1_HUMAN
|
Increases Phosphorylation
|
[7] |
|
Serine-protein kinase ATM (ATM)
|
OTQVOHLT
|
ATM_HUMAN
|
Increases Expression
|
[21] |
|
Baculoviral IAP repeat-containing protein 2 (BIRC2)
|
OTFXFREP
|
BIRC2_HUMAN
|
Increases Expression
|
[21] |
|
Caspase-8 (CASP8)
|
OTA8TVI8
|
CASP8_HUMAN
|
Increases Expression
|
[21] |
|
Angiopoietin-1 (ANGPT1)
|
OTVZ1NG3
|
ANGP1_HUMAN
|
Increases Expression
|
[23] |
|
Bcl-2 homologous antagonist/killer (BAK1)
|
OTDP6ILW
|
BAK_HUMAN
|
Increases Expression
|
[21] |
|
Hypoxia-inducible factor 1-alpha (HIF1A)
|
OTADSC03
|
HIF1A_HUMAN
|
Increases Expression
|
[8] |
|
Small ubiquitin-related modifier 4 (SUMO4)
|
OT9B447E
|
SUMO4_HUMAN
|
Increases Expression
|
[28] |
|
Transient receptor potential cation channel subfamily M member 8 (TRPM8)
|
OT7ACGYK
|
TRPM8_HUMAN
|
Increases Activity
|
[26] |
|
Tumor protein p53-inducible nuclear protein 2 (TP53INP2)
|
OT0GTBXO
|
T53I2_HUMAN
|
Increases Expression
|
[7] |
|
Probable E3 ubiquitin-protein ligase TRIML1 (TRIML1)
|
OTKKSF1S
|
TRIML_HUMAN
|
Increases Expression
|
[7] |
|
Arrestin domain-containing protein 4 (ARRDC4)
|
OTINJ0FX
|
ARRD4_HUMAN
|
Decreases Expression
|
[10] |
|
Transient receptor potential cation channel subfamily V member 1 (TRPV1)
|
OTHHDR03
|
TRPV1_HUMAN
|
Decreases Expression
|
[42] |
|
Folliculin (FLCN)
|
OTVM78XM
|
FLCN_HUMAN
|
Increases Expression
|
[7] |
|
Mitogen-activated protein kinase 15 (MAPK15)
|
OT8SW0L7
|
MK15_HUMAN
|
Increases Expression
|
[7] |
|
Bcl2-associated agonist of cell death (BAD)
|
OT63ERYM
|
BAD_HUMAN
|
Increases Expression
|
[21] |
|
Tumor necrosis factor receptor superfamily member 25 (TNFRSF25)
|
OTVZ7I3A
|
TNR25_HUMAN
|
Increases Expression
|
[21] |
|
Optineurin (OPTN)
|
OT2UXWH9
|
OPTN_HUMAN
|
Increases Expression
|
[7] |
|
Ras-related protein Rab-39B (RAB39B)
|
OTDCLLT0
|
RB39B_HUMAN
|
Increases Expression
|
[7] |
|
NAD-dependent protein deacetylase sirtuin-1 (SIRT1)
|
OTAYZMOY
|
SIR1_HUMAN
|
Affects Expression
|
[57] |
|
Tribbles homolog 3 (TRIB3)
|
OTG5OS7X
|
TRIB3_HUMAN
|
Increases Expression
|
[7] |
|
Growth/differentiation factor 15 (GDF15)
|
OTWQN50N
|
GDF15_HUMAN
|
Increases Expression
|
[58] |
|
TNF receptor-associated factor 4 (TRAF4)
|
OTJLRVMC
|
TRAF4_HUMAN
|
Increases Expression
|
[21] |
|
Serine/threonine-protein kinase PINK1, mitochondrial (PINK1)
|
OT50NR57
|
PINK1_HUMAN
|
Increases Expression
|
[7] |
|
Autophagy protein 5 (ATG5)
|
OT4T5SMS
|
ATG5_HUMAN
|
Increases Expression
|
[37] |
|
Microtubule-associated proteins 1A/1B light chain 3A (MAP1LC3A)
|
OTPMGIU4
|
MLP3A_HUMAN
|
Affects Localization
|
[7] |
|
Ras-related GTP-binding protein C (RRAGC)
|
OTV3XCV8
|
RRAGC_HUMAN
|
Increases Expression
|
[7] |
|
Mucin-5B (MUC5B)
|
OTPW6K5C
|
MUC5B_HUMAN
|
Increases Secretion
|
[59] |
|
NADPH oxidase 4 (NOX4)
|
OTTYQ097
|
NOX4_HUMAN
|
Affects Expression
|
[57] |
|
Ras-related GTP-binding protein D (RRAGD)
|
OTXLVWAH
|
RRAGD_HUMAN
|
Increases Expression
|
[7] |
|
Protein DEPP1 (DEPP1)
|
OTB36PHJ
|
DEPP1_HUMAN
|
Increases Expression
|
[7] |
|
Bcl-2-associated transcription factor 1 (BCLAF1)
|
OT7T8H6A
|
BCLF1_HUMAN
|
Increases Expression
|
[21] |
|
F-box/LRR-repeat protein 2 (FBXL2)
|
OT5EZ7PD
|
FBXL2_HUMAN
|
Increases Expression
|
[7] |
|
Tyrosine-protein phosphatase non-receptor type 22 (PTPN22)
|
OTDCNTC3
|
PTN22_HUMAN
|
Increases Expression
|
[7] |
|
Sestrin-1 (SESN1)
|
OTSFDZWL
|
SESN1_HUMAN
|
Increases Expression
|
[7] |
|
Interleukin-1 beta (IL1B)
|
OT0DWXXB
|
IL1B_HUMAN
|
Increases Response To Substance
|
[60] |
|
NADH-ubiquinone oxidoreductase chain 1 (ND1)
|
OTCLGIXV
|
NU1M_HUMAN
|
Increases Response To Substance
|
[61] |
|
Ecto-NOX disulfide-thiol exchanger 2 (ENOX2)
|
OTMDA97Z
|
ENOX2_HUMAN
|
Increases Response To Substance
|
[62] |
|
GTP cyclohydrolase 1 (GCH1)
|
OTOZ6NSL
|
GCH1_HUMAN
|
Affects Response To Substance
|
[63] |
|
Substance-K receptor (TACR2)
|
OTTQHQZM
|
NK2R_HUMAN
|
Increases Response To Substance
|
[64] |
|
Transient receptor potential cation channel subfamily V member 6 (TRPV6)
|
OTZ0LGNO
|
TRPV6_HUMAN
|
Increases Response To Substance
|
[56] |
|
Endothelin-1 (EDN1)
|
OTZCACEG
|
EDN1_HUMAN
|
Decreases Activity
|
[65] |
| ------------------------------------------------------------------------------------ |
|
|
|
|