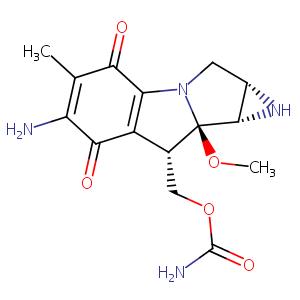
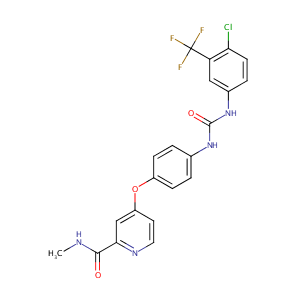
| DOT Name |
DOT ID |
UniProt ID |
Mode of Action |
REF |
|
ATP-dependent translocase ABCB1 (ABCB1)
|
OTEJROBO
|
MDR1_HUMAN
|
Increases Expression
|
[15] |
|
Alternative prion protein (PRNP)
|
OTE85L1Q
|
APRIO_HUMAN
|
Increases Expression
|
[16] |
|
Amyloid-beta precursor protein (APP)
|
OTKFD7R4
|
A4_HUMAN
|
Increases Expression
|
[16] |
|
HLA class I histocompatibility antigen, C alpha chain (HLA-C)
|
OTV38BUJ
|
HLAC_HUMAN
|
Decreases Expression
|
[16] |
|
Bone morphogenetic protein 4 (BMP4)
|
OTPZMDFH
|
BMP4_HUMAN
|
Decreases Expression
|
[16] |
|
G2/mitotic-specific cyclin-B1 (CCNB1)
|
OT19S7E5
|
CCNB1_HUMAN
|
Increases Expression
|
[16] |
|
Early growth response protein 1 (EGR1)
|
OTCP6XGZ
|
EGR1_HUMAN
|
Increases Expression
|
[16] |
|
Cyclic AMP-dependent transcription factor ATF-4 (ATF4)
|
OTRFV19J
|
ATF4_HUMAN
|
Increases Expression
|
[16] |
|
DNA ligase 1 (LIG1)
|
OTEEQS43
|
DNLI1_HUMAN
|
Decreases Expression
|
[16] |
|
Nuclear transcription factor Y subunit alpha (NFYA)
|
OTWFFOVH
|
NFYA_HUMAN
|
Increases Expression
|
[16] |
|
Ephrin type-A receptor 2 (EPHA2)
|
OTI6QNX2
|
EPHA2_HUMAN
|
Decreases Expression
|
[16] |
|
Replication factor C subunit 4 (RFC4)
|
OTWALD2R
|
RFC4_HUMAN
|
Decreases Expression
|
[16] |
|
Matrix metalloproteinase-14 (MMP14)
|
OT9C197Z
|
MMP14_HUMAN
|
Decreases Expression
|
[16] |
|
Activated RNA polymerase II transcriptional coactivator p15 (SUB1)
|
OTK71JYU
|
TCP4_HUMAN
|
Increases Expression
|
[16] |
|
Small ribosomal subunit protein eS1 (RPS3A)
|
OTUEP7CL
|
RS3A_HUMAN
|
Increases Expression
|
[16] |
|
Microtubule-associated protein RP/EB family member 1 (MAPRE1)
|
OTCVQD60
|
MARE1_HUMAN
|
Increases Expression
|
[16] |
|
Histone-binding protein RBBP7 (RBBP7)
|
OTLB56HX
|
RBBP7_HUMAN
|
Increases Expression
|
[16] |
|
Prostaglandin G/H synthase 2 (PTGS2)
|
OT75U9M4
|
PGH2_HUMAN
|
Increases Expression
|
[17] |
|
TP53-regulated inhibitor of apoptosis 1 (TRIAP1)
|
OTEAUJXN
|
TRIA1_HUMAN
|
Increases Expression
|
[18] |
|
Superoxide dismutase (SOD1)
|
OT39TA1L
|
SODC_HUMAN
|
Increases Expression
|
[18] |
|
Cellular tumor antigen p53 (TP53)
|
OTIE1VH3
|
P53_HUMAN
|
Increases Expression
|
[18] |
|
Heat shock cognate 71 kDa protein (HSPA8)
|
OTJI2RCI
|
HSP7C_HUMAN
|
Increases Expression
|
[18] |
|
Proliferating cell nuclear antigen (PCNA)
|
OTHZ1RIA
|
PCNA_HUMAN
|
Increases Expression
|
[18] |
|
Growth arrest and DNA damage-inducible protein GADD45 alpha (GADD45A)
|
OTDRV63V
|
GA45A_HUMAN
|
Increases Expression
|
[18] |
|
Cyclin-dependent kinase inhibitor 1 (CDKN1A)
|
OTQWHCZE
|
CDN1A_HUMAN
|
Increases Expression
|
[18] |
|
Quinone oxidoreductase PIG3 (TP53I3)
|
OTSCM68G
|
QORX_HUMAN
|
Increases Expression
|
[18] |
|
DNA damage-binding protein 2 (DDB2)
|
OTO8HVVB
|
DDB2_HUMAN
|
Increases Expression
|
[18] |
|
Cystine/glutamate transporter (SLC7A11)
|
OTKJ6PXW
|
XCT_HUMAN
|
Decreases Expression
|
[19] |
|
Succinate dehydrogenase assembly factor 1, mitochondrial (SDHAF1)
|
OTDG5VW7
|
SDHF1_HUMAN
|
Decreases Expression
|
[8] |
|
Serine/threonine-protein kinase Sgk1 (SGK1)
|
OT301T1U
|
SGK1_HUMAN
|
Decreases Expression
|
[8] |
|
E3 ubiquitin-protein ligase TRIM38 (TRIM38)
|
OT6SP1M9
|
TRI38_HUMAN
|
Decreases Expression
|
[8] |
|
Torsin-1A (TOR1A)
|
OTYCLGJU
|
TOR1A_HUMAN
|
Decreases Expression
|
[8] |
|
Telomerase reverse transcriptase (TERT)
|
OT085VVA
|
TERT_HUMAN
|
Decreases Expression
|
[20] |
|
Serine/threonine-protein kinase Chk1 (CHEK1)
|
OTTTI622
|
CHK1_HUMAN
|
Increases Phosphorylation
|
[21] |
|
Transcription factor EC (TFEC)
|
OTUST8MR
|
TFEC_HUMAN
|
Decreases Expression
|
[8] |
|
Regulator of G-protein signaling 16 (RGS16)
|
OTBKZASI
|
RGS16_HUMAN
|
Decreases Expression
|
[8] |
|
E3 ubiquitin-protein ligase RNF113A (RNF113A)
|
OTR6N7HU
|
R113A_HUMAN
|
Decreases Expression
|
[8] |
|
HMG box-containing protein 1 (HBP1)
|
OTDPGGDV
|
HBP1_HUMAN
|
Decreases Expression
|
[8] |
|
Kinesin-like protein KIF20A (KIF20A)
|
OTXOQHE0
|
KI20A_HUMAN
|
Decreases Expression
|
[8] |
|
Myelin protein zero-like protein 1 (MPZL1)
|
OTJSUUHR
|
MPZL1_HUMAN
|
Decreases Expression
|
[8] |
|
Serine/threonine-protein kinase Chk2 (CHEK2)
|
OT8ZPCNS
|
CHK2_HUMAN
|
Increases Phosphorylation
|
[21] |
|
Transforming growth factor beta-1 proprotein (TGFB1)
|
OTV5XHVH
|
TGFB1_HUMAN
|
Increases Expression
|
[22] |
|
Interleukin-1 alpha (IL1A)
|
OTPSGILV
|
IL1A_HUMAN
|
Increases Expression
|
[23] |
|
Thymidine kinase, cytosolic (TK1)
|
OTY5JFM1
|
KITH_HUMAN
|
Increases Mutagenesis
|
[24] |
|
Plasminogen activator inhibitor 1 (SERPINE1)
|
OTT0MPQ3
|
PAI1_HUMAN
|
Increases Expression
|
[23] |
|
Interleukin-6 (IL6)
|
OTUOSCCU
|
IL6_HUMAN
|
Increases Expression
|
[23] |
|
Retinoblastoma-associated protein (RB1)
|
OTQJUJMZ
|
RB_HUMAN
|
Decreases Expression
|
[25] |
|
Fibroblast growth factor 2 (FGF2)
|
OT7YUJ9F
|
FGF2_HUMAN
|
Increases Expression
|
[22] |
|
Poly polymerase 1 (PARP1)
|
OT310QSG
|
PARP1_HUMAN
|
Increases Activity
|
[26] |
|
Interleukin-8 (CXCL8)
|
OTS7T5VH
|
IL8_HUMAN
|
Increases Expression
|
[27] |
|
Apoptosis regulator Bcl-2 (BCL2)
|
OT9DVHC0
|
BCL2_HUMAN
|
Increases Expression
|
[28] |
|
C-C motif chemokine 2 (CCL2)
|
OTAD2HEL
|
CCL2_HUMAN
|
Increases Expression
|
[27] |
|
CD59 glycoprotein (CD59)
|
OTSUMQDH
|
CD59_HUMAN
|
Increases Mutagenesis
|
[29] |
|
Histone H2AX (H2AX)
|
OT18UX57
|
H2AX_HUMAN
|
Increases Expression
|
[30] |
|
Beta-galactosidase (GLB1)
|
OTB0TKAG
|
BGAL_HUMAN
|
Increases Activity
|
[23] |
|
rRNA 2'-O-methyltransferase fibrillarin (FBL)
|
OTRODIE5
|
FBRL_HUMAN
|
Affects Localization
|
[31] |
|
Carnitine O-palmitoyltransferase 2, mitochondrial (CPT2)
|
OTIN6G20
|
CPT2_HUMAN
|
Decreases Expression
|
[8] |
|
Tumor necrosis factor receptor superfamily member 6 (FAS)
|
OTP9XG86
|
TNR6_HUMAN
|
Increases Expression
|
[32] |
|
Mitogen-activated protein kinase 3 (MAPK3)
|
OTCYKGKO
|
MK03_HUMAN
|
Increases Phosphorylation
|
[33] |
|
Mitogen-activated protein kinase 1 (MAPK1)
|
OTH85PI5
|
MK01_HUMAN
|
Increases Phosphorylation
|
[33] |
|
GTP cyclohydrolase 1 (GCH1)
|
OTOZ6NSL
|
GCH1_HUMAN
|
Decreases Expression
|
[8] |
|
RAC-alpha serine/threonine-protein kinase (AKT1)
|
OT8H2YY7
|
AKT1_HUMAN
|
Decreases Expression
|
[34] |
|
Replication factor C subunit 1 (RFC1)
|
OT3L5PK3
|
RFC1_HUMAN
|
Decreases Expression
|
[8] |
|
Caspase-3 (CASP3)
|
OTIJRBE7
|
CASP3_HUMAN
|
Increases Activity
|
[35] |
|
RNA-binding protein 34 (RBM34)
|
OTX3LUGI
|
RBM34_HUMAN
|
Decreases Expression
|
[8] |
|
Tumor necrosis factor ligand superfamily member 6 (FASLG)
|
OTZARCHH
|
TNFL6_HUMAN
|
Increases Expression
|
[32] |
|
Glycogen synthase kinase-3 beta (GSK3B)
|
OTL3L14B
|
GSK3B_HUMAN
|
Increases Phosphorylation
|
[23] |
|
LIM/homeobox protein Lhx2 (LHX2)
|
OTK61NP8
|
LHX2_HUMAN
|
Decreases Expression
|
[8] |
|
DNA mismatch repair protein Msh6 (MSH6)
|
OT46FP09
|
MSH6_HUMAN
|
Decreases Expression
|
[8] |
|
RecQ-like DNA helicase BLM (BLM)
|
OTEJOAJX
|
BLM_HUMAN
|
Decreases Expression
|
[8] |
|
Ataxin-1 (ATXN1)
|
OTQF0HNR
|
ATX1_HUMAN
|
Decreases Expression
|
[8] |
|
Caspase-9 (CASP9)
|
OTD4RFFG
|
CASP9_HUMAN
|
Increases Activity
|
[34] |
|
Ras-related protein Rap-2b (RAP2B)
|
OTD2NDQP
|
RAP2B_HUMAN
|
Decreases Expression
|
[8] |
|
Cytochrome c (CYCS)
|
OTBFALJD
|
CYC_HUMAN
|
Increases Secretion
|
[35] |
|
E3 ubiquitin-protein ligase Mdm2 (MDM2)
|
OTOVXARF
|
MDM2_HUMAN
|
Increases Expression
|
[36] |
|
Transcription factor E2F1 (E2F1)
|
OTLKYBBC
|
E2F1_HUMAN
|
Decreases Expression
|
[8] |
|
Transcription factor p65 (RELA)
|
OTUJP9CN
|
TF65_HUMAN
|
Increases Expression
|
[37] |
|
Protein kinase C zeta type (PRKCZ)
|
OTN2FE42
|
KPCZ_HUMAN
|
Increases Cleavage
|
[35] |
|
DNA repair protein RAD51 homolog 1 (RAD51)
|
OTNVWGC1
|
RAD51_HUMAN
|
Increases Expression
|
[33] |
|
Apoptosis regulator BAX (BAX)
|
OTAW0V4V
|
BAX_HUMAN
|
Decreases Expression
|
[28] |
|
Bcl-2-like protein 1 (BCL2L1)
|
OTRC5K9O
|
B2CL1_HUMAN
|
Decreases Expression
|
[38] |
|
Tripartite motif-containing protein 26 (TRIM26)
|
OTS0DJIP
|
TRI26_HUMAN
|
Decreases Expression
|
[8] |
|
E3 ubiquitin-protein ligase TRIM32 (TRIM32)
|
OTJOV0PG
|
TRI32_HUMAN
|
Decreases Expression
|
[8] |
|
Chromatin assembly factor 1 subunit A (CHAF1A)
|
OTXSSY4H
|
CAF1A_HUMAN
|
Decreases Expression
|
[8] |
|
Chromatin assembly factor 1 subunit B (CHAF1B)
|
OTOMK4KH
|
CAF1B_HUMAN
|
Decreases Expression
|
[8] |
|
Interleukin-18 (IL18)
|
OTBB2A8O
|
IL18_HUMAN
|
Decreases Expression
|
[37] |
|
Protein BTG3 (BTG3)
|
OT9ANHVT
|
BTG3_HUMAN
|
Decreases Expression
|
[8] |
|
Histone RNA hairpin-binding protein (SLBP)
|
OTVYYQRT
|
SLBP_HUMAN
|
Decreases Expression
|
[8] |
|
Caspase-8 (CASP8)
|
OTA8TVI8
|
CASP8_HUMAN
|
Increases Activity
|
[35] |
|
Transforming growth factor-beta-induced protein ig-h3 (TGFBI)
|
OTR443C5
|
BGH3_HUMAN
|
Decreases Expression
|
[38] |
|
Neutral amino acid transporter B(0) (SLC1A5)
|
OTE2H26Q
|
AAAT_HUMAN
|
Decreases Expression
|
[8] |
|
Protein MCM10 homolog (MCM10)
|
OTV0O3JN
|
MCM10_HUMAN
|
Decreases Expression
|
[8] |
|
Partner and localizer of BRCA2 (PALB2)
|
OT6DNDBG
|
PALB2_HUMAN
|
Decreases Expression
|
[8] |
|
Leucine-rich repeat-containing protein 47 (LRRC47)
|
OTSE8NAO
|
LRC47_HUMAN
|
Decreases Expression
|
[8] |
|
Bcl2-associated agonist of cell death (BAD)
|
OT63ERYM
|
BAD_HUMAN
|
Increases Expression
|
[34] |
|
Cytochrome c oxidase assembly factor 7 (COA7)
|
OTRQJYL6
|
COA7_HUMAN
|
Decreases Expression
|
[8] |
|
Mitochondrial potassium channel (CCDC51)
|
OTOJNHNA
|
MITOK_HUMAN
|
Decreases Expression
|
[8] |
|
Integrator complex subunit 15 (INTS15)
|
OT3O4INM
|
INT15_HUMAN
|
Decreases Expression
|
[8] |
|
Krueppel-like factor 6 (KLF6)
|
OTQY9S7F
|
KLF6_HUMAN
|
Decreases Expression
|
[8] |
|
Cell division control protein 6 homolog (CDC6)
|
OTX93FE7
|
CDC6_HUMAN
|
Decreases Expression
|
[8] |
|
Growth/differentiation factor 15 (GDF15)
|
OTWQN50N
|
GDF15_HUMAN
|
Increases Expression
|
[8] |
|
Protein orai-3 (ORAI3)
|
OTUP3OH3
|
ORAI3_HUMAN
|
Decreases Expression
|
[8] |
|
Protein pelota homolog (PELO)
|
OTF3WLIM
|
PELO_HUMAN
|
Decreases Expression
|
[8] |
|
TIMELESS-interacting protein (TIPIN)
|
OT9PZHXV
|
TIPIN_HUMAN
|
Decreases Expression
|
[8] |
|
Fanconi anemia group D2 protein (FANCD2)
|
OTVEB5LF
|
FACD2_HUMAN
|
Increases Ubiquitination
|
[21] |
|
tRNA pseudouridine(38/39) synthase (PUS3)
|
OT6WG6M2
|
PUS3_HUMAN
|
Decreases Expression
|
[8] |
|
Transmembrane protein 185B (TMEM185B)
|
OTGNYS0J
|
T185B_HUMAN
|
Decreases Expression
|
[8] |
|
Interferon-stimulated 20 kDa exonuclease-like 2 (ISG20L2)
|
OT3Q11WQ
|
I20L2_HUMAN
|
Decreases Expression
|
[8] |
|
GrpE protein homolog 1, mitochondrial (GRPEL1)
|
OT8O00YJ
|
GRPE1_HUMAN
|
Decreases Expression
|
[8] |
|
Forkhead box protein J2 (FOXJ2)
|
OTPT639J
|
FOXJ2_HUMAN
|
Decreases Expression
|
[8] |
|
Homologous-pairing protein 2 homolog (PSMC3IP)
|
OT9UB5UO
|
HOP2_HUMAN
|
Decreases Expression
|
[8] |
|
rRNA methyltransferase 2, mitochondrial (MRM2)
|
OTRYA1HU
|
MRM2_HUMAN
|
Decreases Expression
|
[8] |
|
Frizzled-1 (FZD1)
|
OTZATHVS
|
FZD1_HUMAN
|
Decreases Expression
|
[8] |
|
Interleukin-1 beta (IL1B)
|
OT0DWXXB
|
IL1B_HUMAN
|
Affects Response To Substance
|
[11] |
|
Tripartite motif-containing protein 2 (TRIM2)
|
OT0V1YVC
|
TRIM2_HUMAN
|
Affects Response To Substance
|
[11] |
|
Acid ceramidase (ASAH1)
|
OT1DNGXL
|
ASAH1_HUMAN
|
Affects Response To Substance
|
[11] |
|
Fanconi-associated nuclease 1 (FAN1)
|
OT1LM1HZ
|
FAN1_HUMAN
|
Increases Response To Substance
|
[39] |
|
Replication factor C subunit 3 (RFC3)
|
OT1MS7AO
|
RFC3_HUMAN
|
Affects Response To Substance
|
[11] |
|
CD9 antigen (CD9)
|
OT2184XU
|
CD9_HUMAN
|
Affects Response To Substance
|
[11] |
|
KN motif and ankyrin repeat domain-containing protein 1 (KANK1)
|
OT2E7A6W
|
KANK1_HUMAN
|
Affects Response To Substance
|
[11] |
|
Aldehyde oxidase (AOX1)
|
OT2FZP6H
|
AOXA_HUMAN
|
Affects Response To Substance
|
[11] |
|
Mitochondrial fission 1 protein (FIS1)
|
OT2HL10J
|
FIS1_HUMAN
|
Affects Response To Substance
|
[11] |
|
Cytoplasmic aconitate hydratase (ACO1)
|
OT2VUR7L
|
ACOHC_HUMAN
|
Affects Response To Substance
|
[11] |
|
Syncytin-2 (ERVFRD-1)
|
OT3064D7
|
SYCY2_HUMAN
|
Affects Response To Substance
|
[11] |
|
Protein FAM171A1 (FAM171A1)
|
OT33BHEP
|
F1711_HUMAN
|
Affects Response To Substance
|
[11] |
|
Sister chromatid cohesion protein PDS5 homolog A (PDS5A)
|
OT34P56Z
|
PDS5A_HUMAN
|
Affects Response To Substance
|
[11] |
|
Excitatory amino acid transporter 3 (SLC1A1)
|
OT3B9M6W
|
EAA3_HUMAN
|
Affects Response To Substance
|
[11] |
|
Syntabulin (SYBU)
|
OT3FQV7N
|
SYBU_HUMAN
|
Affects Response To Substance
|
[11] |
|
Fatty acid CoA ligase Acsl3 (ACSL3)
|
OT3MWER1
|
ACSL3_HUMAN
|
Affects Response To Substance
|
[11] |
|
Prelamin-A/C (LMNA)
|
OT3SG7ZR
|
LMNA_HUMAN
|
Affects Response To Substance
|
[11] |
|
Methylated-DNA--protein-cysteine methyltransferase (MGMT)
|
OT40A9WH
|
MGMT_HUMAN
|
Increases Response To Substance
|
[40] |
|
Pyridoxal kinase (PDXK)
|
OT40AJ5J
|
PDXK_HUMAN
|
Affects Response To Substance
|
[11] |
|
Proteinase-activated receptor 1 (F2R)
|
OT4WVWBO
|
PAR1_HUMAN
|
Affects Response To Substance
|
[11] |
|
Diamine acetyltransferase 1 (SAT1)
|
OT52AU22
|
SAT1_HUMAN
|
Affects Response To Substance
|
[11] |
|
Breast cancer type 1 susceptibility protein (BRCA1)
|
OT5BN6VH
|
BRCA1_HUMAN
|
Increases Response To Substance
|
[41] |
|
Neprilysin (MME)
|
OT5Q39P8
|
NEP_HUMAN
|
Affects Response To Substance
|
[11] |
|
Lymphokine-activated killer T-cell-originated protein kinase (PBK)
|
OT5Z27TW
|
TOPK_HUMAN
|
Affects Response To Substance
|
[11] |
|
Protein-glutamine gamma-glutamyltransferase 2 (TGM2)
|
OT6MFOWF
|
TGM2_HUMAN
|
Affects Response To Substance
|
[11] |
|
G-protein-signaling modulator 2 (GPSM2)
|
OT6RPMRM
|
GPSM2_HUMAN
|
Affects Response To Substance
|
[11] |
|
Dual specificity protein phosphatase 4 (DUSP4)
|
OT6WAO12
|
DUS4_HUMAN
|
Affects Response To Substance
|
[11] |
|
Interferon-induced GTP-binding protein Mx1 (MX1)
|
OT6X8G5T
|
MX1_HUMAN
|
Increases Response To Substance
|
[42] |
|
Plasminogen activator inhibitor 2 (SERPINB2)
|
OT72QLZB
|
PAI2_HUMAN
|
Affects Response To Substance
|
[11] |
|
Transcriptional regulator ATRX (ATRX)
|
OT77RSQW
|
ATRX_HUMAN
|
Affects Response To Substance
|
[11] |
|
Monocarboxylate transporter 7 (SLC16A6)
|
OT7BIIZX
|
MOT7_HUMAN
|
Affects Response To Substance
|
[11] |
|
Ras-related protein Rab-17 (RAB17)
|
OT7NBUGQ
|
RAB17_HUMAN
|
Affects Response To Substance
|
[11] |
|
Stanniocalcin-2 (STC2)
|
OT86YP8G
|
STC2_HUMAN
|
Affects Response To Substance
|
[11] |
|
Pirin (PIR)
|
OT8ALXHU
|
PIR_HUMAN
|
Affects Response To Substance
|
[11] |
|
Myelin proteolipid protein (PLP1)
|
OT8CM9CX
|
MYPR_HUMAN
|
Affects Response To Substance
|
[11] |
|
Protein-S-isoprenylcysteine O-methyltransferase (ICMT)
|
OT8CNKBO
|
ICMT_HUMAN
|
Affects Response To Substance
|
[11] |
|
G0/G1 switch protein 2 (G0S2)
|
OT8FL49L
|
G0S2_HUMAN
|
Affects Response To Substance
|
[11] |
|
Serine/threonine-protein phosphatase 2A 56 kDa regulatory subunit epsilon isoform (PPP2R5E)
|
OT8GPFT5
|
2A5E_HUMAN
|
Decreases Response To Substance
|
[43] |
|
cAMP-dependent protein kinase inhibitor alpha (PKIA)
|
OT8M5GVO
|
IPKA_HUMAN
|
Affects Response To Substance
|
[11] |
|
Melanoma-associated antigen 12 (MAGEA12)
|
OT8ULELL
|
MAGAC_HUMAN
|
Affects Response To Substance
|
[11] |
|
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B alpha isoform (PPP2R2A)
|
OT9297OG
|
2ABA_HUMAN
|
Decreases Response To Substance
|
[43] |
|
Tissue factor pathway inhibitor (TFPI)
|
OTA0FX16
|
TFPI1_HUMAN
|
Affects Response To Substance
|
[11] |
|
Acyl-coenzyme A thioesterase 2, mitochondrial (ACOT2)
|
OTAKUO27
|
ACOT2_HUMAN
|
Affects Response To Substance
|
[11] |
|
Aldehyde dehydrogenase, dimeric NADP-preferring (ALDH3A1)
|
OTAYZZE6
|
AL3A1_HUMAN
|
Affects Response To Substance
|
[44] |
|
Long-chain-fatty-acid--CoA ligase 1 (ACSL1)
|
OTB06ESI
|
ACSL1_HUMAN
|
Affects Response To Substance
|
[11] |
|
Cyclin-dependent kinase 2 (CDK2)
|
OTB5DYYZ
|
CDK2_HUMAN
|
Affects Response To Substance
|
[11] |
|
Cochlin (COCH)
|
OTBEHD89
|
COCH_HUMAN
|
Affects Response To Substance
|
[11] |
|
ETS domain-containing protein Elk-3 (ELK3)
|
OTBM9BHT
|
ELK3_HUMAN
|
Affects Response To Substance
|
[11] |
|
High mobility group protein HMGI-C (HMGA2)
|
OTBMBHHL
|
HMGA2_HUMAN
|
Affects Response To Substance
|
[11] |
|
Protein PRRC2C (PRRC2C)
|
OTBX3MXM
|
PRC2C_HUMAN
|
Affects Response To Substance
|
[11] |
|
Melanocyte protein PMEL (PMEL)
|
OTCDDHHM
|
PMEL_HUMAN
|
Affects Response To Substance
|
[11] |
|
BMP and activin membrane-bound inhibitor homolog (BAMBI)
|
OTCEJ8W5
|
BAMBI_HUMAN
|
Affects Response To Substance
|
[11] |
|
Creatine kinase U-type, mitochondrial (CKMT1A)
|
OTCINHH5
|
KCRU_HUMAN
|
Affects Response To Substance
|
[11] |
|
Sodium/potassium-transporting ATPase subunit alpha-1 (ATP1A1)
|
OTCJ458Q
|
AT1A1_HUMAN
|
Affects Response To Substance
|
[11] |
|
CD166 antigen (ALCAM)
|
OTCO4LP7
|
CD166_HUMAN
|
Affects Response To Substance
|
[11] |
|
Cytochrome c oxidase copper chaperone (COX17)
|
OTCP4LH2
|
COX17_HUMAN
|
Affects Response To Substance
|
[11] |
|
Interleukin-6 receptor subunit alpha (IL6R)
|
OTCQL07Z
|
IL6RA_HUMAN
|
Decreases Response To Substance
|
[45] |
|
Collagen alpha-3(IX) chain (COL9A3)
|
OTCUJOEK
|
CO9A3_HUMAN
|
Affects Response To Substance
|
[11] |
|
A-kinase anchor protein 12 (AKAP12)
|
OTCVRDDX
|
AKA12_HUMAN
|
Affects Response To Substance
|
[11] |
|
Dystrophin (DMD)
|
OTD21T5J
|
DMD_HUMAN
|
Affects Response To Substance
|
[11] |
|
Dickkopf-related protein 3 (DKK3)
|
OTDU94EY
|
DKK3_HUMAN
|
Affects Response To Substance
|
[11] |
|
dTDP-D-glucose 4,6-dehydratase (TGDS)
|
OTEOFLOS
|
TGDS_HUMAN
|
Affects Response To Substance
|
[11] |
|
Calmegin (CLGN)
|
OTEWVFQV
|
CLGN_HUMAN
|
Affects Response To Substance
|
[11] |
|
Ras association domain-containing protein 1 (RASSF1)
|
OTEZIPB7
|
RASF1_HUMAN
|
Increases Response To Substance
|
[46] |
|
Protocadherin Fat 1 (FAT1)
|
OTF174DH
|
FAT1_HUMAN
|
Affects Response To Substance
|
[11] |
|
Midkine (MDK)
|
OTF24HKC
|
MK_HUMAN
|
Affects Response To Substance
|
[11] |
|
Serine/arginine-rich splicing factor 1 (SRSF1)
|
OTF61HOV
|
SRSF1_HUMAN
|
Affects Response To Substance
|
[11] |
|
Serine/threonine-protein phosphatase 2A 56 kDa regulatory subunit gamma isoform (PPP2R5C)
|
OTF7CGO2
|
2A5G_HUMAN
|
Increases Response To Substance
|
[43] |
|
G antigen 7 (GAGE7)
|
OTF8BTR9
|
GAGE7_HUMAN
|
Affects Response To Substance
|
[11] |
|
Serglycin (SRGN)
|
OTFAVSX0
|
SRGN_HUMAN
|
Affects Response To Substance
|
[11] |
|
Catenin alpha-1 (CTNNA1)
|
OTFC725Z
|
CTNA1_HUMAN
|
Affects Response To Substance
|
[11] |
|
Sortilin (SORT1)
|
OTFCQ4B1
|
SORT_HUMAN
|
Affects Response To Substance
|
[11] |
|
Baculoviral IAP repeat-containing protein 2 (BIRC2)
|
OTFXFREP
|
BIRC2_HUMAN
|
Affects Response To Substance
|
[11] |
|
Myelin basic protein (MBP)
|
OTFZCEDB
|
MBP_HUMAN
|
Affects Response To Substance
|
[11] |
|
Tudor domain-containing protein 3 (TDRD3)
|
OTG83E2C
|
TDRD3_HUMAN
|
Affects Response To Substance
|
[11] |
|
Enhancer of filamentation 1 (NEDD9)
|
OTGCFN4M
|
CASL_HUMAN
|
Affects Response To Substance
|
[11] |
|
Squalene synthase (FDFT1)
|
OTGDISIT
|
FDFT_HUMAN
|
Affects Response To Substance
|
[11] |
|
Multidrug resistance-associated protein 1 (ABCC1)
|
OTGUN89S
|
MRP1_HUMAN
|
Decreases Response To Substance
|
[47] |
|
Sigma intracellular receptor 2 (TMEM97)
|
OTH76ZWK
|
SGMR2_HUMAN
|
Affects Response To Substance
|
[11] |
|
Transmembrane protein 156 (TMEM156)
|
OTH7YJID
|
TM156_HUMAN
|
Affects Response To Substance
|
[11] |
|
Probable JmjC domain-containing histone demethylation protein 2C (JMJD1C)
|
OTHAWK8C
|
JHD2C_HUMAN
|
Affects Response To Substance
|
[11] |
|
Unconventional myosin-X (MYO10)
|
OTHB78ZQ
|
MYO10_HUMAN
|
Affects Response To Substance
|
[11] |
|
Tribbles homolog 2 (TRIB2)
|
OTHSX3MX
|
TRIB2_HUMAN
|
Affects Response To Substance
|
[11] |
|
Actin-binding LIM protein 1 (ABLIM1)
|
OTHXEK3E
|
ABLM1_HUMAN
|
Affects Response To Substance
|
[11] |
|
DNA damage-inducible transcript 4 protein (DDIT4)
|
OTHY8SY4
|
DDIT4_HUMAN
|
Affects Response To Substance
|
[11] |
|
Protein S100-A11 (S100A11)
|
OTI57KDN
|
S10AB_HUMAN
|
Affects Response To Substance
|
[11] |
|
Protein S100-A10 (S100A10)
|
OTI71243
|
S10AA_HUMAN
|
Affects Response To Substance
|
[11] |
|
Melanoma-associated antigen 6 (MAGEA6)
|
OTI8S0O2
|
MAGA6_HUMAN
|
Affects Response To Substance
|
[11] |
|
Runt-related transcription factor 3 (RUNX3)
|
OTITK1XD
|
RUNX3_HUMAN
|
Increases Response To Substance
|
[48] |
|
Superoxide dismutase , mitochondrial (SOD2)
|
OTIWXGZ9
|
SODM_HUMAN
|
Affects Response To Substance
|
[11] |
|
Nuclear receptor-interacting protein 1 (NRIP1)
|
OTIZOJQV
|
NRIP1_HUMAN
|
Affects Response To Substance
|
[11] |
|
Protein c-Fos (FOS)
|
OTJBUVWS
|
FOS_HUMAN
|
Affects Response To Substance
|
[11] |
|
Trefoil factor 3 (TFF3)
|
OTJJDRTU
|
TFF3_HUMAN
|
Affects Response To Substance
|
[11] |
|
Collagen alpha-2(IV) chain (COL4A2)
|
OTJK1LKN
|
CO4A2_HUMAN
|
Affects Response To Substance
|
[11] |
|
Hairy/enhancer-of-split related with YRPW motif protein 1 (HEY1)
|
OTJQL0I3
|
HEY1_HUMAN
|
Affects Response To Substance
|
[11] |
|
E3 ubiquitin-protein ligase MYLIP (MYLIP)
|
OTL0PFGV
|
MYLIP_HUMAN
|
Affects Response To Substance
|
[11] |
|
Hematopoietically-expressed homeobox protein HHEX (HHEX)
|
OTLIUVYX
|
HHEX_HUMAN
|
Affects Response To Substance
|
[11] |
|
Glycylpeptide N-tetradecanoyltransferase 2 (NMT2)
|
OTLNA39Z
|
NMT2_HUMAN
|
Affects Response To Substance
|
[11] |
|
Glutathione S-transferase P (GSTP1)
|
OTLP0A0Y
|
GSTP1_HUMAN
|
Increases Response To Substance
|
[49] |
|
Protein S100-A4 (S100A4)
|
OTLRGFSQ
|
S10A4_HUMAN
|
Affects Response To Substance
|
[11] |
|
Tyrosine-protein kinase Fyn (FYN)
|
OTLSLVZS
|
FYN_HUMAN
|
Affects Response To Substance
|
[11] |
|
Bridging integrator 3 (BIN3)
|
OTMIWWDW
|
BIN3_HUMAN
|
Affects Response To Substance
|
[11] |
|
Transcription factor AP-2-alpha (TFAP2A)
|
OTMYT3NK
|
AP2A_HUMAN
|
Affects Response To Substance
|
[11] |
|
Four and a half LIM domains protein 1 (FHL1)
|
OTN535SU
|
FHL1_HUMAN
|
Affects Response To Substance
|
[11] |
|
Cysteine-rich hydrophobic domain-containing protein 2 (CHIC2)
|
OTNE3T6Z
|
CHIC2_HUMAN
|
Affects Response To Substance
|
[11] |
|
Inositol monophosphatase 2 (IMPA2)
|
OTNFB9YN
|
IMPA2_HUMAN
|
Affects Response To Substance
|
[11] |
|
Neural cell adhesion molecule L1 (L1CAM)
|
OTNWAQ4Y
|
L1CAM_HUMAN
|
Affects Response To Substance
|
[11] |
|
Cyclin-dependent kinase inhibitor 1B (CDKN1B)
|
OTNY5LLZ
|
CDN1B_HUMAN
|
Affects Response To Substance
|
[50] |
|
Kynurenine--oxoglutarate transaminase 3 (KYAT3)
|
OTO4U2QK
|
KAT3_HUMAN
|
Affects Response To Substance
|
[11] |
|
Beta-secretase 2 (BACE2)
|
OTO5YQVK
|
BACE2_HUMAN
|
Affects Response To Substance
|
[11] |
|
Histone deacetylase 9 (HDAC9)
|
OTO8O0LF
|
HDAC9_HUMAN
|
Affects Response To Substance
|
[11] |
|
SH3 domain-binding protein 5 (SH3BP5)
|
OTOOEGUJ
|
3BP5_HUMAN
|
Affects Response To Substance
|
[11] |
|
Glutathione S-transferase Mu 4 (GSTM4)
|
OTOTF8DU
|
GSTM4_HUMAN
|
Affects Response To Substance
|
[11] |
|
Ras-related protein Rab-20 (RAB20)
|
OTP9OOVS
|
RAB20_HUMAN
|
Affects Response To Substance
|
[11] |
|
Tumor protein D52 (TPD52)
|
OTPKSK43
|
TPD52_HUMAN
|
Affects Response To Substance
|
[11] |
|
DNA dC->dU-editing enzyme APOBEC-3C (APOBEC3C)
|
OTPL0AI1
|
ABC3C_HUMAN
|
Affects Response To Substance
|
[11] |
|
Kazrin (KAZN)
|
OTPM7BYM
|
KAZRN_HUMAN
|
Affects Response To Substance
|
[11] |
|
DNA-binding protein inhibitor ID-4 (ID4)
|
OTPMJ39I
|
ID4_HUMAN
|
Affects Response To Substance
|
[11] |
|
Centrosomal protein of 57 kDa (CEP57)
|
OTPOHLIX
|
CEP57_HUMAN
|
Affects Response To Substance
|
[11] |
|
Epithelial membrane protein 2 (EMP2)
|
OTPS2H0L
|
EMP2_HUMAN
|
Affects Response To Substance
|
[11] |
|
Dermatan-sulfate epimerase (DSE)
|
OTQ108VJ
|
DSE_HUMAN
|
Affects Response To Substance
|
[11] |
|
Adhesion G-protein coupled receptor G1 (ADGRG1)
|
OTQBB8NT
|
AGRG1_HUMAN
|
Affects Response To Substance
|
[11] |
|
Cyclic GMP-AMP phosphodiesterase SMPDL3A (SMPDL3A)
|
OTQDYH8E
|
ASM3A_HUMAN
|
Affects Response To Substance
|
[11] |
|
MAPK/MAK/MRK overlapping kinase (MOK)
|
OTQK7M9V
|
MOK_HUMAN
|
Affects Response To Substance
|
[11] |
|
High mobility group protein HMG-I/HMG-Y (HMGA1)
|
OTQUSHPX
|
HMGA1_HUMAN
|
Affects Response To Substance
|
[11] |
|
Receptor tyrosine-protein kinase erbB-3 (ERBB3)
|
OTRSST0A
|
ERBB3_HUMAN
|
Affects Response To Substance
|
[11] |
|
Dr1-associated corepressor (DRAP1)
|
OTRWLF6D
|
NC2A_HUMAN
|
Affects Response To Substance
|
[11] |
|
Latent-transforming growth factor beta-binding protein 2 (LTBP2)
|
OTS88GSD
|
LTBP2_HUMAN
|
Affects Response To Substance
|
[11] |
|
Terminal nucleotidyltransferase 5A (TENT5A)
|
OTSYF511
|
TET5A_HUMAN
|
Affects Response To Substance
|
[11] |
|
Lipopolysaccharide-induced tumor necrosis factor-alpha factor (LITAF)
|
OTT5JX1F
|
LITAF_HUMAN
|
Affects Response To Substance
|
[11] |
|
DNA repair protein RAD51 homolog 3 (RAD51C)
|
OTUD6SY5
|
RA51C_HUMAN
|
Increases Response To Substance
|
[51] |
|
Calcium-dependent secretion activator 2 (CADPS2)
|
OTV1FW0M
|
CAPS2_HUMAN
|
Affects Response To Substance
|
[11] |
|
Matrilin-2 (MATN2)
|
OTVDR68G
|
MATN2_HUMAN
|
Affects Response To Substance
|
[11] |
|
NADPH--cytochrome P450 reductase (POR)
|
OTVIDOCH
|
NCPR_HUMAN
|
Increases Response To Substance
|
[49] |
|
Ensconsin (MAP7)
|
OTVSSJ33
|
MAP7_HUMAN
|
Affects Response To Substance
|
[11] |
|
Protein artemis (DCLRE1C)
|
OTW3KB1I
|
DCR1C_HUMAN
|
Decreases Response To Substance
|
[12] |
|
Sorbin and SH3 domain-containing protein 1 (SORBS1)
|
OTWH8762
|
SRBS1_HUMAN
|
Affects Response To Substance
|
[11] |
|
Protein mago nashi homolog (MAGOH)
|
OTWRYTOX
|
MGN_HUMAN
|
Affects Response To Substance
|
[11] |
|
Urokinase-type plasminogen activator (PLAU)
|
OTX0QGKK
|
UROK_HUMAN
|
Affects Response To Substance
|
[11] |
|
Acylamino-acid-releasing enzyme (APEH)
|
OTX258QE
|
ACPH_HUMAN
|
Affects Response To Substance
|
[11] |
|
Hematopoietic lineage cell-specific protein (HCLS1)
|
OTX7WGYN
|
HCLS1_HUMAN
|
Affects Response To Substance
|
[11] |
|
S-adenosylhomocysteine hydrolase-like protein 1 (AHCYL1)
|
OTX8L3M5
|
SAHH2_HUMAN
|
Affects Response To Substance
|
[11] |
|
Long-chain fatty acid transport protein 3 (SLC27A3)
|
OTXB60L7
|
S27A3_HUMAN
|
Affects Response To Substance
|
[11] |
|
Metallothionein-1E (MT1E)
|
OTXJKU4Y
|
MT1E_HUMAN
|
Affects Response To Substance
|
[11] |
|
Arginine vasopressin-induced protein 1 (AVPI1)
|
OTXSBR60
|
AVPI1_HUMAN
|
Affects Response To Substance
|
[11] |
|
Neuroendocrine protein 7B2 (SCG5)
|
OTXSJMT1
|
7B2_HUMAN
|
Affects Response To Substance
|
[11] |
|
Carbamoyl-phosphate synthase , mitochondrial (CPS1)
|
OTXV8NSR
|
CPSM_HUMAN
|
Affects Response To Substance
|
[11] |
|
Peripheral myelin protein 22 (PMP22)
|
OTXWYWCZ
|
PMP22_HUMAN
|
Affects Response To Substance
|
[11] |
|
Kinesin-like protein KIF23 (KIF23)
|
OTY850JC
|
KIF23_HUMAN
|
Affects Response To Substance
|
[11] |
|
SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily E member 1-related (HMG20B)
|
OTY94WRA
|
HM20B_HUMAN
|
Affects Response To Substance
|
[11] |
|
Protein Niban 1 (NIBAN1)
|
OTYOLI12
|
NIBA1_HUMAN
|
Affects Response To Substance
|
[11] |
|
LIM and calponin homology domains-containing protein 1 (LIMCH1)
|
OTYPC4XA
|
LIMC1_HUMAN
|
Affects Response To Substance
|
[11] |
|
Galectin-3 (LGALS3)
|
OTYQT0ZI
|
LEG3_HUMAN
|
Affects Response To Substance
|
[11] |
|
L-dopachrome tautomerase (DCT)
|
OTYVNTBG
|
TYRP2_HUMAN
|
Affects Response To Substance
|
[11] |
|
Epithelial discoidin domain-containing receptor 1 (DDR1)
|
OTYW1SH8
|
DDR1_HUMAN
|
Affects Response To Substance
|
[11] |
|
Tissue factor pathway inhibitor 2 (TFPI2)
|
OTZCRWOR
|
TFPI2_HUMAN
|
Affects Response To Substance
|
[11] |
|
Suppressor of tumorigenicity 7 protein (ST7)
|
OTZG8RC6
|
ST7_HUMAN
|
Affects Response To Substance
|
[11] |
|
NAD(P)H dehydrogenase 1 (NQO1)
|
OTZGGIVK
|
NQO1_HUMAN
|
Increases Response To Substance
|
[49] |
|
CTD small phosphatase-like protein (CTDSPL)
|
OTZJ0CZK
|
CTDSL_HUMAN
|
Affects Response To Substance
|
[11] |
|
C-X-C motif chemokine 5 (CXCL5)
|
OTZOUPCA
|
CXCL5_HUMAN
|
Affects Response To Substance
|
[11] |
|
Eukaryotic translation initiation factor 5B (EIF5B)
|
OTZTT22W
|
IF2P_HUMAN
|
Affects Response To Substance
|
[11] |
| ------------------------------------------------------------------------------------ |
|
|
|
|