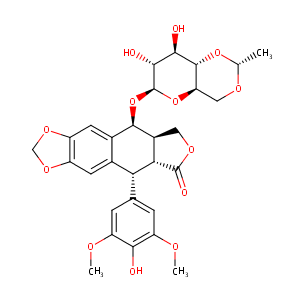
| DOT Name |
DOT ID |
UniProt ID |
Mode of Action |
REF |
|
Prostaglandin G/H synthase 2 (PTGS2)
|
OT75U9M4
|
PGH2_HUMAN
|
Increases Expression
|
[42] |
|
Cytochrome P450 3A4 (CYP3A4)
|
OTQGYY83
|
CP3A4_HUMAN
|
Increases Expression
|
[43] |
|
Cytochrome P450 1A2 (CYP1A2)
|
OTLLBX48
|
CP1A2_HUMAN
|
Increases Expression
|
[43] |
|
Cytochrome P450 3A5 (CYP3A5)
|
OTSXFBXB
|
CP3A5_HUMAN
|
Decreases Methylation
|
[44] |
|
Glutathione S-transferase P (GSTP1)
|
OTLP0A0Y
|
GSTP1_HUMAN
|
Decreases Response To Substance
|
[45] |
|
ATP-dependent translocase ABCB1 (ABCB1)
|
OTEJROBO
|
MDR1_HUMAN
|
Increases Expression
|
[46] |
|
ATP-binding cassette sub-family C member 3 (ABCC3)
|
OTC3IJV4
|
MRP3_HUMAN
|
Decreases Response To Substance
|
[47] |
|
Multidrug resistance-associated protein 1 (ABCC1)
|
OTGUN89S
|
MRP1_HUMAN
|
Decreases Response To Substance
|
[45] |
|
ATP-binding cassette sub-family C member 2 (ABCC2)
|
OTJSIGV5
|
MRP2_HUMAN
|
Affects Transport
|
[48] |
|
DNA topoisomerase 2-alpha (TOP2A)
|
OT6LPS08
|
TOP2A_HUMAN
|
Decreases Response To Substance
|
[49] |
|
Mitogen-activated protein kinase 1 (MAPK1)
|
OTH85PI5
|
MK01_HUMAN
|
Increases Phosphorylation
|
[50] |
|
Caspase-3 (CASP3)
|
OTIJRBE7
|
CASP3_HUMAN
|
Increases Activity
|
[51] |
|
Apoptosis regulator Bcl-2 (BCL2)
|
OT9DVHC0
|
BCL2_HUMAN
|
Increases ADR
|
[52] |
|
Caspase-8 (CASP8)
|
OTA8TVI8
|
CASP8_HUMAN
|
Decreases Response To Substance
|
[53] |
|
Tumor protein p73 (TP73)
|
OT0LUO47
|
P73_HUMAN
|
Increases Expression
|
[9] |
|
25-hydroxyvitamin D-1 alpha hydroxylase, mitochondrial (CYP27B1)
|
OTTK98BH
|
CP27B_HUMAN
|
Increases Expression
|
[9] |
|
Dihydropyrimidinase-related protein 4 (DPYSL4)
|
OT3SBS2S
|
DPYL4_HUMAN
|
Increases Expression
|
[10] |
|
Copper chaperone for superoxide dismutase (CCS)
|
OTXHT3QO
|
CCS_HUMAN
|
Increases Expression
|
[10] |
|
Urokinase-type plasminogen activator (PLAU)
|
OTX0QGKK
|
UROK_HUMAN
|
Increases Expression
|
[10] |
|
Myc proto-oncogene protein (MYC)
|
OTPV5LUK
|
MYC_HUMAN
|
Decreases Expression
|
[10] |
|
Ubiquitin-like protein ISG15 (ISG15)
|
OT53QQ7N
|
ISG15_HUMAN
|
Increases Expression
|
[10] |
|
Integrin alpha-IIb (ITGA2B)
|
OT4Y17PY
|
ITA2B_HUMAN
|
Increases Expression
|
[10] |
|
Ras-related protein R-Ras (RRAS)
|
OTBBF28C
|
RRAS_HUMAN
|
Increases Expression
|
[10] |
|
Growth arrest and DNA damage-inducible protein GADD45 alpha (GADD45A)
|
OTDRV63V
|
GA45A_HUMAN
|
Increases Expression
|
[10] |
|
Stomatin (STOM)
|
OTC8R6EH
|
STOM_HUMAN
|
Increases Expression
|
[10] |
|
Cellular retinoic acid-binding protein 2 (CRABP2)
|
OTY01V9G
|
RABP2_HUMAN
|
Increases Expression
|
[10] |
|
Tyrosine-protein kinase receptor UFO (AXL)
|
OTKA2SUX
|
UFO_HUMAN
|
Increases Expression
|
[10] |
|
14-3-3 protein sigma (SFN)
|
OTLJCZ1U
|
1433S_HUMAN
|
Increases Expression
|
[10] |
|
Galectin-7 (LGALS7)
|
OTMSVI7R
|
LEG7_HUMAN
|
Increases Expression
|
[10] |
|
Rho GDP-dissociation inhibitor 2 (ARHGDIB)
|
OT9PD6CS
|
GDIR2_HUMAN
|
Increases Expression
|
[10] |
|
Epithelial membrane protein 1 (EMP1)
|
OTSZHUHQ
|
EMP1_HUMAN
|
Increases Expression
|
[10] |
|
Epithelial membrane protein 3 (EMP3)
|
OTODMJ1D
|
EMP3_HUMAN
|
Increases Expression
|
[10] |
|
Protein ripply3 (RIPPLY3)
|
OT1HK35I
|
DSCR6_HUMAN
|
Increases Expression
|
[10] |
|
Kinesin-like protein KIF1A (KIF1A)
|
OT3JVEGV
|
KIF1A_HUMAN
|
Increases Expression
|
[10] |
|
Filamin-C (FLNC)
|
OT3F8J6Y
|
FLNC_HUMAN
|
Increases Expression
|
[10] |
|
Immediate early response gene 5 protein (IER5)
|
OTJPTXMD
|
IER5_HUMAN
|
Increases Expression
|
[10] |
|
Keratin, type II cytoskeletal 80 (KRT80)
|
OTAU54U3
|
K2C80_HUMAN
|
Increases Expression
|
[10] |
|
All-trans-retinol 13,14-reductase (RETSAT)
|
OTC3AOPX
|
RETST_HUMAN
|
Increases Expression
|
[10] |
|
Mitochondria-eating protein (SPATA18)
|
OTOEHTHU
|
MIEAP_HUMAN
|
Increases Expression
|
[10] |
|
Tumor necrosis factor receptor superfamily member 14 (TNFRSF14)
|
OTB82PFO
|
TNR14_HUMAN
|
Increases Expression
|
[10] |
|
Major histocompatibility complex class I-related gene protein (MR1)
|
OTZU3XX7
|
HMR1_HUMAN
|
Increases Expression
|
[10] |
|
Roundabout homolog 3 (ROBO3)
|
OTPVG40S
|
ROBO3_HUMAN
|
Increases Expression
|
[10] |
|
Neuronal PAS domain-containing protein 1 (NPAS1)
|
OTZKO6ZN
|
NPAS1_HUMAN
|
Increases Expression
|
[10] |
|
Ankyrin repeat domain-containing protein 10 (ANKRD10)
|
OTIZ2VK8
|
ANR10_HUMAN
|
Decreases Expression
|
[10] |
|
Serine/threonine-protein kinase PLK2 (PLK2)
|
OTKMJXJ8
|
PLK2_HUMAN
|
Increases Expression
|
[10] |
|
Solute carrier family 2, facilitated glucose transporter member 6 (SLC2A6)
|
OTVUZCLG
|
GTR6_HUMAN
|
Increases Expression
|
[10] |
|
Glutaminase liver isoform, mitochondrial (GLS2)
|
OT08MSHL
|
GLSL_HUMAN
|
Increases Expression
|
[10] |
|
Endothelial protein C receptor (PROCR)
|
OTRHED17
|
EPCR_HUMAN
|
Increases Expression
|
[10] |
|
Fatty acid desaturase 3 (FADS3)
|
OT9RVXGE
|
FADS3_HUMAN
|
Increases Expression
|
[10] |
|
Catalase (CAT)
|
OTHEBX9R
|
CATA_HUMAN
|
Increases Activity
|
[54] |
|
NADH-ubiquinone oxidoreductase chain 4 (ND4)
|
OT4RQVAA
|
NU4M_HUMAN
|
Increases Expression
|
[42] |
|
Nitric oxide synthase, inducible (NOS2)
|
OTKKIOJ1
|
NOS2_HUMAN
|
Increases Expression
|
[42] |
|
Transcription factor A, mitochondrial (TFAM)
|
OTXXV5V7
|
TFAM_HUMAN
|
Increases Expression
|
[42] |
|
TP53-binding protein 1 (TP53BP1)
|
OTL8879S
|
TP53B_HUMAN
|
Increases Expression
|
[42] |
|
Peroxisome proliferator-activated receptor gamma coactivator 1-beta (PPARGC1B)
|
OTN6FKCB
|
PRGC2_HUMAN
|
Increases Expression
|
[42] |
|
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PPARGC1A)
|
OTHCDQ22
|
PRGC1_HUMAN
|
Increases Expression
|
[42] |
|
Calsequestrin-2 (CASQ2)
|
OT09MNQ8
|
CASQ2_HUMAN
|
Decreases Expression
|
[11] |
|
Telethonin (TCAP)
|
OTQQMJ94
|
TELT_HUMAN
|
Decreases Expression
|
[11] |
|
LIM domain-binding protein 3 (LDB3)
|
OTGQL1AM
|
LDB3_HUMAN
|
Decreases Expression
|
[11] |
|
Creatine kinase M-type (CKM)
|
OTME0KO7
|
KCRM_HUMAN
|
Decreases Expression
|
[11] |
|
Glutathione peroxidase 1 (GPX1)
|
OTE2O72Q
|
GPX1_HUMAN
|
Increases Expression
|
[11] |
|
Myosin light chain 3 (MYL3)
|
OTKD3RSX
|
MYL3_HUMAN
|
Decreases Expression
|
[11] |
|
Keratin, type I cytoskeletal 19 (KRT19)
|
OTDGDQ75
|
K1C19_HUMAN
|
Increases Expression
|
[11] |
|
Calmodulin-1 (CALM2)
|
OTNYA92F
|
CALM1_HUMAN
|
Increases Expression
|
[11] |
|
Myosin regulatory light chain 2, ventricular/cardiac muscle isoform (MYL2)
|
OT78PC0C
|
MLRV_HUMAN
|
Decreases Expression
|
[11] |
|
Glycogen phosphorylase, muscle form (PYGM)
|
OTWLVWTV
|
PYGM_HUMAN
|
Decreases Expression
|
[11] |
|
Dystrophin (DMD)
|
OTD21T5J
|
DMD_HUMAN
|
Decreases Expression
|
[11] |
|
Myosin-7 (MYH7)
|
OT4Z9T8N
|
MYH7_HUMAN
|
Decreases Expression
|
[11] |
|
Leukemia inhibitory factor (LIF)
|
OTO46S5S
|
LIF_HUMAN
|
Increases Expression
|
[11] |
|
NAD(P)H dehydrogenase 1 (NQO1)
|
OTZGGIVK
|
NQO1_HUMAN
|
Increases Expression
|
[11] |
|
Ankyrin-1 (ANK1)
|
OTU79URK
|
ANK1_HUMAN
|
Increases Expression
|
[11] |
|
Desmin (DES)
|
OTI09KBW
|
DESM_HUMAN
|
Decreases Expression
|
[11] |
|
Tumor necrosis factor receptor superfamily member 6 (FAS)
|
OTP9XG86
|
TNR6_HUMAN
|
Increases Expression
|
[11] |
|
CCN family member 2 (CCN2)
|
OTC39NSU
|
CCN2_HUMAN
|
Increases Expression
|
[11] |
|
Sodium/calcium exchanger 1 (SLC8A1)
|
OT787VRN
|
NAC1_HUMAN
|
Decreases Expression
|
[11] |
|
Thrombospondin-4 (THBS4)
|
OTA1T9KK
|
TSP4_HUMAN
|
Increases Expression
|
[11] |
|
Gap junction alpha-5 protein (GJA5)
|
OTRMZVO6
|
CXA5_HUMAN
|
Decreases Expression
|
[11] |
|
Vasopressin V1a receptor (AVPR1A)
|
OTKR8AFL
|
V1AR_HUMAN
|
Increases Expression
|
[11] |
|
Sodium/potassium-transporting ATPase subunit alpha-2 (ATP1A2)
|
OTCF8OWW
|
AT1A2_HUMAN
|
Decreases Expression
|
[11] |
|
cAMP-dependent protein kinase catalytic subunit PRKX (PRKX)
|
OTDAC6Z0
|
PRKX_HUMAN
|
Increases Expression
|
[11] |
|
Stanniocalcin-1 (STC1)
|
OTGVVXYF
|
STC1_HUMAN
|
Increases Expression
|
[11] |
|
Myomesin-2 (MYOM2)
|
OTD2UOXW
|
MYOM2_HUMAN
|
Decreases Expression
|
[11] |
|
Actin, alpha skeletal muscle (ACTA1)
|
OTOVGLPG
|
ACTS_HUMAN
|
Decreases Expression
|
[11] |
|
Iroquois-class homeodomain protein IRX-4 (IRX4)
|
OT0TV6WK
|
IRX4_HUMAN
|
Decreases Expression
|
[11] |
|
Arginase-2, mitochondrial (ARG2)
|
OTCPI0E8
|
ARGI2_HUMAN
|
Decreases Expression
|
[11] |
|
Cytochrome c oxidase subunit 6A2, mitochondrial (COX6A2)
|
OTQR5SXD
|
CX6A2_HUMAN
|
Decreases Expression
|
[11] |
|
Pro-neuregulin-1, membrane-bound isoform (NRG1)
|
OTZO6F1X
|
NRG1_HUMAN
|
Increases Expression
|
[11] |
|
Dual specificity protein phosphatase 4 (DUSP4)
|
OT6WAO12
|
DUS4_HUMAN
|
Increases Expression
|
[11] |
|
Krueppel-like factor 5 (KLF5)
|
OT1ABI9N
|
KLF5_HUMAN
|
Increases Expression
|
[11] |
|
Four and a half LIM domains protein 2 (FHL2)
|
OT0OAYWT
|
FHL2_HUMAN
|
Decreases Expression
|
[11] |
|
Transmembrane glycoprotein NMB (GPNMB)
|
OTB7KQKV
|
GPNMB_HUMAN
|
Increases Expression
|
[11] |
|
Periostin (POSTN)
|
OTWP62UA
|
POSTN_HUMAN
|
Increases Expression
|
[11] |
|
kinase isozyme 1, mitochondrial (PDK1)
|
OTMABK9Z
|
PDK1_HUMAN
|
Decreases Expression
|
[11] |
|
kinase isozyme 4, mitochondrial (PDK4)
|
OTCMHMBZ
|
PDK4_HUMAN
|
Increases Expression
|
[11] |
|
PDZ and LIM domain protein 3 (PDLIM3)
|
OTVXQC81
|
PDLI3_HUMAN
|
Increases Expression
|
[11] |
|
Nebulin-related-anchoring protein (NRAP)
|
OTO6H3YF
|
NRAP_HUMAN
|
Decreases Expression
|
[11] |
|
Sodium channel subunit beta-4 (SCN4B)
|
OT3JSUWO
|
SCN4B_HUMAN
|
Increases Expression
|
[11] |
|
Actin-binding Rho-activating protein (ABRA)
|
OTBQ2C1J
|
ABRA_HUMAN
|
Increases Expression
|
[11] |
|
Transient receptor potential cation channel subfamily V member 1 (TRPV1)
|
OTHHDR03
|
TRPV1_HUMAN
|
Increases Expression
|
[11] |
|
Ryanodine receptor 2 (RYR2)
|
OT0PF19E
|
RYR2_HUMAN
|
Decreases Expression
|
[11] |
|
Small conductance calcium-activated potassium channel protein 2 (KCNN2)
|
OTQVTMK5
|
KCNN2_HUMAN
|
Decreases Expression
|
[11] |
|
Regulator of cell cycle RGCC (RGCC)
|
OTYJMLWM
|
RGCC_HUMAN
|
Increases Expression
|
[11] |
|
Myotilin (MYOT)
|
OTCEW5XW
|
MYOTI_HUMAN
|
Increases Expression
|
[11] |
|
Protein ATP1B4 (ATP1B4)
|
OT2YC9GZ
|
AT1B4_HUMAN
|
Increases Expression
|
[11] |
|
Junctional adhesion molecule A (F11R)
|
OTGHPAV6
|
JAM1_HUMAN
|
Increases Expression
|
[11] |
|
Succinate dehydrogenase assembly factor 1, mitochondrial (SDHAF1)
|
OTDG5VW7
|
SDHF1_HUMAN
|
Decreases Expression
|
[13] |
|
Agrin (AGRN)
|
OTWJENAZ
|
AGRIN_HUMAN
|
Increases Expression
|
[12] |
|
E3 ubiquitin-protein ligase TRIM38 (TRIM38)
|
OT6SP1M9
|
TRI38_HUMAN
|
Decreases Expression
|
[13] |
|
Torsin-1A (TOR1A)
|
OTYCLGJU
|
TOR1A_HUMAN
|
Decreases Expression
|
[13] |
|
Etoposide-induced protein 2.4 homolog (EI24)
|
OTD4NOYS
|
EI24_HUMAN
|
Increases Expression
|
[12] |
|
Tumor necrosis factor receptor superfamily member 10B (TNFRSF10B)
|
OTA1CPBV
|
TR10B_HUMAN
|
Increases Expression
|
[55] |
|
Tax1-binding protein 3 (TAX1BP3)
|
OTQ6IB4T
|
TX1B3_HUMAN
|
Increases Expression
|
[12] |
|
Inhibitor of nuclear factor kappa-B kinase subunit beta (IKBKB)
|
OT9RDS3H
|
IKKB_HUMAN
|
Decreases Expression
|
[56] |
|
Proepiregulin (EREG)
|
OTRM4NQY
|
EREG_HUMAN
|
Increases Expression
|
[57] |
|
Transcription factor EC (TFEC)
|
OTUST8MR
|
TFEC_HUMAN
|
Decreases Expression
|
[13] |
|
Inhibitor of nuclear factor kappa-B kinase subunit alpha (CHUK)
|
OTLF4ZB1
|
IKKA_HUMAN
|
Decreases Expression
|
[56] |
|
Protein phosphatase 1D (PPM1D)
|
OT8NLZ9D
|
PPM1D_HUMAN
|
Increases Expression
|
[58] |
|
Baculoviral IAP repeat-containing protein 5 (BIRC5)
|
OTILXZYL
|
BIRC5_HUMAN
|
Increases Expression
|
[59] |
|
CASP8 and FADD-like apoptosis regulator (CFLAR)
|
OTX14BAS
|
CFLAR_HUMAN
|
Increases Expression
|
[60] |
|
E3 ubiquitin-protein ligase RNF113A (RNF113A)
|
OTR6N7HU
|
R113A_HUMAN
|
Decreases Expression
|
[13] |
|
Receptor-interacting serine/threonine-protein kinase 2 (RIPK2)
|
OT0KMIYQ
|
RIPK2_HUMAN
|
Increases Expression
|
[59] |
|
Krueppel-like factor 4 (KLF4)
|
OT4O9RQW
|
KLF4_HUMAN
|
Increases Expression
|
[61] |
|
Forkhead box protein O3 (FOXO3)
|
OTHXQG4P
|
FOXO3_HUMAN
|
Increases Phosphorylation
|
[62] |
|
Cytoplasmic protein NCK2 (NCK2)
|
OTUYPF55
|
NCK2_HUMAN
|
Decreases Expression
|
[12] |
|
Protein regulator of cytokinesis 1 (PRC1)
|
OTHD0XS0
|
PRC1_HUMAN
|
Decreases Expression
|
[12] |
|
Mitotic checkpoint serine/threonine-protein kinase BUB1 (BUB1)
|
OT80DZMT
|
BUB1_HUMAN
|
Decreases Expression
|
[63] |
|
HMG box-containing protein 1 (HBP1)
|
OTDPGGDV
|
HBP1_HUMAN
|
Decreases Expression
|
[13] |
|
Mitotic checkpoint serine/threonine-protein kinase BUB1 beta (BUB1B)
|
OT8KME51
|
BUB1B_HUMAN
|
Decreases Expression
|
[63] |
|
Cell division control protein 45 homolog (CDC45)
|
OT6NNLOD
|
CDC45_HUMAN
|
Decreases Expression
|
[63] |
|
Nuclear receptor subfamily 1 group I member 2 (NR1I2)
|
OTC5U0N5
|
NR1I2_HUMAN
|
Increases Activity
|
[64] |
|
Kinesin-like protein KIF20A (KIF20A)
|
OTXOQHE0
|
KI20A_HUMAN
|
Decreases Expression
|
[13] |
|
Myelin protein zero-like protein 1 (MPZL1)
|
OTJSUUHR
|
MPZL1_HUMAN
|
Decreases Expression
|
[13] |
|
Serine/threonine-protein kinase Chk2 (CHEK2)
|
OT8ZPCNS
|
CHK2_HUMAN
|
Increases Phosphorylation
|
[65] |
|
Aldehyde dehydrogenase 1A1 (ALDH1A1)
|
OTCUWZKB
|
AL1A1_HUMAN
|
Increases Expression
|
[61] |
|
Epidermal growth factor receptor (EGFR)
|
OTAPLO1S
|
EGFR_HUMAN
|
Decreases Expression
|
[57] |
|
Protein c-Fos (FOS)
|
OTJBUVWS
|
FOS_HUMAN
|
Increases Expression
|
[66] |
|
Protransforming growth factor alpha (TGFA)
|
OTPD1LL9
|
TGFA_HUMAN
|
Increases Expression
|
[57] |
|
HLA class I histocompatibility antigen, B alpha chain (HLA-B)
|
OTNXFWY2
|
HLAB_HUMAN
|
Affects Expression
|
[67] |
|
Transferrin receptor protein 1 (TFRC)
|
OT8ZPBDL
|
TFR1_HUMAN
|
Decreases Expression
|
[61] |
|
Ferritin light chain (FTL)
|
OTYQA8A6
|
FRIL_HUMAN
|
Increases Expression
|
[61] |
|
Metallothionein-2 (MT2A)
|
OTHOACHD
|
MT2_HUMAN
|
Decreases Expression
|
[16] |
|
Interstitial collagenase (MMP1)
|
OTI4I2V1
|
MMP1_HUMAN
|
Decreases Expression
|
[68] |
|
Superoxide dismutase , mitochondrial (SOD2)
|
OTIWXGZ9
|
SODM_HUMAN
|
Increases Expression
|
[51] |
|
HLA class I histocompatibility antigen, A alpha chain (HLA-A)
|
OTAH14LU
|
HLAA_HUMAN
|
Affects Expression
|
[67] |
|
Receptor tyrosine-protein kinase erbB-2 (ERBB2)
|
OTOAUNCK
|
ERBB2_HUMAN
|
Decreases Expression
|
[57] |
|
Cellular tumor antigen p53 (TP53)
|
OTIE1VH3
|
P53_HUMAN
|
Increases Acetylation
|
[69] |
|
Cytochrome P450 1A1 (CYP1A1)
|
OTE4EFH8
|
CP1A1_HUMAN
|
Increases Expression
|
[43] |
|
Retinoblastoma-associated protein (RB1)
|
OTQJUJMZ
|
RB_HUMAN
|
Decreases Phosphorylation
|
[70] |
|
Transcription factor Sp1 (SP1)
|
OTISPT4X
|
SP1_HUMAN
|
Increases Phosphorylation
|
[71] |
|
High mobility group protein B1 (HMGB1)
|
OT4B7CPF
|
HMGB1_HUMAN
|
Increases Secretion
|
[72] |
|
Annexin A4 (ANXA4)
|
OTUCRYXL
|
ANXA4_HUMAN
|
Increases Expression
|
[12] |
|
Poly polymerase 1 (PARP1)
|
OT310QSG
|
PARP1_HUMAN
|
Increases Cleavage
|
[73] |
|
Interleukin-8 (CXCL8)
|
OTS7T5VH
|
IL8_HUMAN
|
Increases Expression
|
[74] |
|
C-C motif chemokine 3 (CCL3)
|
OTW2H3ND
|
CCL3_HUMAN
|
Increases Expression
|
[74] |
|
Androgen receptor (AR)
|
OTUBKAZZ
|
ANDR_HUMAN
|
Increases Degradation
|
[75] |
|
Cholesteryl ester transfer protein (CETP)
|
OTAGPPOE
|
CETP_HUMAN
|
Increases Expression
|
[76] |
|
Carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1)
|
OTPWI88U
|
CEAM1_HUMAN
|
Increases Expression
|
[12] |
|
Translationally-controlled tumor protein (TPT1)
|
OTHMTRJU
|
TCTP_HUMAN
|
Increases Expression
|
[77] |
|
Tissue factor (F3)
|
OT3MSU3B
|
TF_HUMAN
|
Increases Expression
|
[78] |
|
HLA class I histocompatibility antigen, alpha chain E (HLA-E)
|
OTX1CTFB
|
HLAE_HUMAN
|
Affects Expression
|
[67] |
|
G2/mitotic-specific cyclin-B1 (CCNB1)
|
OT19S7E5
|
CCNB1_HUMAN
|
Decreases Expression
|
[63] |
|
Protein C-ets-1 (ETS1)
|
OT4LVGDN
|
ETS1_HUMAN
|
Increases Expression
|
[56] |
|
Amphiregulin (AREG)
|
OTJFOR67
|
AREG_HUMAN
|
Increases Expression
|
[57] |
|
Histone H2AX (H2AX)
|
OT18UX57
|
H2AX_HUMAN
|
Increases Expression
|
[79] |
|
Syndecan-1 (SDC1)
|
OTWKRVZC
|
SDC1_HUMAN
|
Increases Expression
|
[12] |
|
Cyclin-A2 (CCNA2)
|
OTPHHYZJ
|
CCNA2_HUMAN
|
Decreases Expression
|
[63] |
|
Receptor tyrosine-protein kinase erbB-3 (ERBB3)
|
OTRSST0A
|
ERBB3_HUMAN
|
Decreases Expression
|
[57] |
|
rRNA 2'-O-methyltransferase fibrillarin (FBL)
|
OTRODIE5
|
FBRL_HUMAN
|
Affects Localization
|
[80] |
|
NADPH:adrenodoxin oxidoreductase, mitochondrial (FDXR)
|
OTPDCK1F
|
ADRO_HUMAN
|
Increases Expression
|
[12] |
|
Brain-derived neurotrophic factor (BDNF)
|
OTLGH7EW
|
BDNF_HUMAN
|
Increases Expression
|
[81] |
|
Carnitine O-palmitoyltransferase 2, mitochondrial (CPT2)
|
OTIN6G20
|
CPT2_HUMAN
|
Decreases Expression
|
[13] |
|
G1/S-specific cyclin-D1 (CCND1)
|
OT8HPTKJ
|
CCND1_HUMAN
|
Increases Expression
|
[63] |
|
Cyclin-dependent kinase 2 (CDK2)
|
OTB5DYYZ
|
CDK2_HUMAN
|
Increases Expression
|
[14] |
|
NF-kappa-B inhibitor alpha (NFKBIA)
|
OTFT924M
|
IKBA_HUMAN
|
Increases Degradation
|
[82] |
|
Mitogen-activated protein kinase 3 (MAPK3)
|
OTCYKGKO
|
MK03_HUMAN
|
Increases Phosphorylation
|
[50] |
|
Tripeptidyl-peptidase 2 (TPP2)
|
OT91OVAU
|
TPP2_HUMAN
|
Affects Localization
|
[83] |
|
M-phase inducer phosphatase 1 (CDC25A)
|
OTSLKKCO
|
MPIP1_HUMAN
|
Decreases Expression
|
[13] |
|
M-phase inducer phosphatase 3 (CDC25C)
|
OTPQI71S
|
MPIP3_HUMAN
|
Decreases Expression
|
[63] |
|
Dual specificity protein kinase TTK (TTK)
|
OTBY1Z5H
|
TTK_HUMAN
|
Decreases Expression
|
[63] |
|
DNA replication licensing factor MCM7 (MCM7)
|
OT6FXC6K
|
MCM7_HUMAN
|
Decreases Expression
|
[63] |
|
Replication factor C subunit 1 (RFC1)
|
OT3L5PK3
|
RFC1_HUMAN
|
Decreases Expression
|
[13] |
|
Tumor necrosis factor receptor superfamily member 3 (LTBR)
|
OT2HVZGV
|
TNR3_HUMAN
|
Increases Expression
|
[59] |
|
Breast cancer type 1 susceptibility protein (BRCA1)
|
OT5BN6VH
|
BRCA1_HUMAN
|
Decreases Expression
|
[84] |
|
Cyclin-dependent kinase inhibitor 1 (CDKN1A)
|
OTQWHCZE
|
CDN1A_HUMAN
|
Increases Expression
|
[63] |
|
Transcription factor ETV6 (ETV6)
|
OTCZMG61
|
ETV6_HUMAN
|
Increases Mutagenesis
|
[85] |
|
Tumor necrosis factor ligand superfamily member 9 (TNFSF9)
|
OTV9L89D
|
TNFL9_HUMAN
|
Increases Expression
|
[12] |
|
Serine/threonine-protein kinase mTOR (MTOR)
|
OTHH8KU7
|
MTOR_HUMAN
|
Increases Phosphorylation
|
[86] |
|
Caspase-2 (CASP2)
|
OTUDYSPP
|
CASP2_HUMAN
|
Increases Expression
|
[87] |
|
RNA-binding protein 34 (RBM34)
|
OTX3LUGI
|
RBM34_HUMAN
|
Decreases Expression
|
[13] |
|
DNA repair protein RAD52 homolog (RAD52)
|
OT0OTDHI
|
RAD52_HUMAN
|
Increases Expression
|
[88] |
|
Cyclin-dependent kinase inhibitor 1B (CDKN1B)
|
OTNY5LLZ
|
CDN1B_HUMAN
|
Increases Expression
|
[62] |
|
Dual specificity mitogen-activated protein kinase kinase 3 (MAP2K3)
|
OTI2OREX
|
MP2K3_HUMAN
|
Increases Expression
|
[12] |
|
Tumor necrosis factor ligand superfamily member 6 (FASLG)
|
OTZARCHH
|
TNFL6_HUMAN
|
Increases Activity
|
[89] |
|
Stromal cell-derived factor 1 (CXCL12)
|
OT2QX5LL
|
SDF1_HUMAN
|
Decreases Expression
|
[90] |
|
Iron-responsive element-binding protein 2 (IREB2)
|
OT747D24
|
IREB2_HUMAN
|
Decreases Expression
|
[61] |
|
Nuclear receptor-interacting protein 1 (NRIP1)
|
OTIZOJQV
|
NRIP1_HUMAN
|
Decreases Expression
|
[76] |
|
Fatty acid synthase (FASN)
|
OTFII9KG
|
FAS_HUMAN
|
Increases Expression
|
[91] |
|
Centromere protein F (CENPF)
|
OT7AG0SW
|
CENPF_HUMAN
|
Decreases Expression
|
[63] |
|
CCAAT/enhancer-binding protein alpha (CEBPA)
|
OTOM9OE4
|
CEBPA_HUMAN
|
Decreases Expression
|
[92] |
|
DNA replication licensing factor MCM2 (MCM2)
|
OTGGORIQ
|
MCM2_HUMAN
|
Decreases Expression
|
[63] |
|
LIM/homeobox protein Lhx2 (LHX2)
|
OTK61NP8
|
LHX2_HUMAN
|
Decreases Expression
|
[13] |
|
Serine/threonine-protein kinase Nek2 (NEK2)
|
OT1H27HO
|
NEK2_HUMAN
|
Decreases Expression
|
[63] |
|
DNA mismatch repair protein Msh6 (MSH6)
|
OT46FP09
|
MSH6_HUMAN
|
Decreases Expression
|
[13] |
|
Death-associated protein kinase 1 (DAPK1)
|
OTNCNUCO
|
DAPK1_HUMAN
|
Increases Expression
|
[56] |
|
Mitogen-activated protein kinase 12 (MAPK12)
|
OTTCKE27
|
MK12_HUMAN
|
Increases Activity
|
[93] |
|
RecQ-like DNA helicase BLM (BLM)
|
OTEJOAJX
|
BLM_HUMAN
|
Decreases Expression
|
[13] |
|
Ataxin-1 (ATXN1)
|
OTQF0HNR
|
ATX1_HUMAN
|
Decreases Expression
|
[13] |
|
Oxysterols receptor LXR-beta (NR1H2)
|
OT4APA60
|
NR1H2_HUMAN
|
Affects Localization
|
[76] |
|
Caspase-7 (CASP7)
|
OTAPJ040
|
CASP7_HUMAN
|
Increases Activity
|
[94] |
|
Caspase-9 (CASP9)
|
OTD4RFFG
|
CASP9_HUMAN
|
Increases Activity
|
[94] |
|
BH3-interacting domain death agonist (BID)
|
OTOSHSHU
|
BID_HUMAN
|
Increases Expression
|
[59] |
|
Triosephosphate isomerase (TPI1)
|
OT14KP4B
|
TPIS_HUMAN
|
Increases Phosphorylation
|
[95] |
|
Small ribosomal subunit protein eS6 (RPS6)
|
OTT4D1LN
|
RS6_HUMAN
|
Increases Phosphorylation
|
[96] |
|
DNA-dependent protein kinase catalytic subunit (PRKDC)
|
OTJVS4EG
|
PRKDC_HUMAN
|
Increases Phosphorylation
|
[71] |
|
Protein BTG2 (BTG2)
|
OTZF6K1H
|
BTG2_HUMAN
|
Increases Expression
|
[13] |
|
Hepcidin (HAMP)
|
OT607RBL
|
HEPC_HUMAN
|
Decreases Expression
|
[97] |
|
Cytochrome c (CYCS)
|
OTBFALJD
|
CYC_HUMAN
|
Affects Localization
|
[51] |
|
E3 ubiquitin-protein ligase Mdm2 (MDM2)
|
OTOVXARF
|
MDM2_HUMAN
|
Increases Expression
|
[63] |
|
Runt-related transcription factor 1 (RUNX1)
|
OTU7J84H
|
RUNX1_HUMAN
|
Increases Mutagenesis
|
[85] |
|
Myelin transcription factor 1 (MYT1)
|
OTC3660I
|
MYT1_HUMAN
|
Decreases Expression
|
[63] |
|
POU domain, class 5, transcription factor 1 (POU5F1)
|
OTDHHN7O
|
PO5F1_HUMAN
|
Increases Expression
|
[61] |
|
DNA topoisomerase 2-beta (TOP2B)
|
OTDHKQZ8
|
TOP2B_HUMAN
|
Decreases Activity
|
[98] |
|
Histone-lysine N-methyltransferase 2A (KMT2A)
|
OT9GLJI6
|
KMT2A_HUMAN
|
Increases Cleavage
|
[99] |
|
Antigen peptide transporter 1 (TAP1)
|
OTJL27PW
|
TAP1_HUMAN
|
Increases Expression
|
[12] |
|
CCAAT/enhancer-binding protein zeta (CEBPZ)
|
OT11BATG
|
CEBPZ_HUMAN
|
Decreases Expression
|
[12] |
|
Apoptosis regulator BAX (BAX)
|
OTAW0V4V
|
BAX_HUMAN
|
Increases Expression
|
[63] |
|
Induced myeloid leukemia cell differentiation protein Mcl-1 (MCL1)
|
OT2YYI1A
|
MCL1_HUMAN
|
Decreases Expression
|
[62] |
|
Forkhead box protein O1 (FOXO1)
|
OTPJRB6D
|
FOXO1_HUMAN
|
Increases Phosphorylation
|
[62] |
|
Chromatin assembly factor 1 subunit A (CHAF1A)
|
OTXSSY4H
|
CAF1A_HUMAN
|
Decreases Expression
|
[13] |
|
Oxysterols receptor LXR-alpha (NR1H3)
|
OT54YZ9I
|
NR1H3_HUMAN
|
Increases Expression
|
[76] |
|
Serine-protein kinase ATM (ATM)
|
OTQVOHLT
|
ATM_HUMAN
|
Increases Phosphorylation
|
[100] |
|
DNA topoisomerase 3-alpha (TOP3A)
|
OT3CKUI9
|
TOP3A_HUMAN
|
Decreases Expression
|
[13] |
|
Baculoviral IAP repeat-containing protein 3 (BIRC3)
|
OT3E95KB
|
BIRC3_HUMAN
|
Decreases Expression
|
[63] |
|
Baculoviral IAP repeat-containing protein 2 (BIRC2)
|
OTFXFREP
|
BIRC2_HUMAN
|
Increases Expression
|
[101] |
|
Phorbol-12-myristate-13-acetate-induced protein 1 (PMAIP1)
|
OTXEE550
|
APR_HUMAN
|
Increases Expression
|
[53] |
|
Protein BTG3 (BTG3)
|
OT9ANHVT
|
BTG3_HUMAN
|
Decreases Expression
|
[13] |
|
Histone RNA hairpin-binding protein (SLBP)
|
OTVYYQRT
|
SLBP_HUMAN
|
Decreases Expression
|
[13] |
|
Hypermethylated in cancer 1 protein (HIC1)
|
OTI9TWY4
|
HIC1_HUMAN
|
Increases Expression
|
[102] |
|
DNA replication licensing factor MCM6 (MCM6)
|
OTKLIAWT
|
MCM6_HUMAN
|
Decreases Expression
|
[63] |
|
Receptor tyrosine-protein kinase erbB-4 (ERBB4)
|
OT9L6C41
|
ERBB4_HUMAN
|
Decreases Expression
|
[57] |
|
Neutral amino acid transporter B(0) (SLC1A5)
|
OTE2H26Q
|
AAAT_HUMAN
|
Decreases Expression
|
[13] |
|
Mothers against decapentaplegic homolog 2 (SMAD2)
|
OTC6VB4K
|
SMAD2_HUMAN
|
Increases Phosphorylation
|
[90] |
|
Cyclin-G2 (CCNG2)
|
OTII38K2
|
CCNG2_HUMAN
|
Increases Expression
|
[103] |
|
Cyclin-dependent kinase inhibitor 3 (CDKN3)
|
OTBE3H07
|
CDKN3_HUMAN
|
Decreases Expression
|
[63] |
|
Quinone oxidoreductase PIG3 (TP53I3)
|
OTSCM68G
|
QORX_HUMAN
|
Increases Expression
|
[12] |
|
Ribosomal protein eS27-like (RPS27L)
|
OTZWHU0N
|
RS27L_HUMAN
|
Increases Expression
|
[104] |
|
Protein MCM10 homolog (MCM10)
|
OTV0O3JN
|
MCM10_HUMAN
|
Decreases Expression
|
[13] |
|
EPM2A-interacting protein 1 (EPM2AIP1)
|
OT21ALNF
|
EPMIP_HUMAN
|
Increases Expression
|
[12] |
|
Ribonucleoside-diphosphate reductase subunit M2 B (RRM2B)
|
OTE8GBUR
|
RIR2B_HUMAN
|
Increases Expression
|
[105] |
|
Partner and localizer of BRCA2 (PALB2)
|
OT6DNDBG
|
PALB2_HUMAN
|
Decreases Expression
|
[13] |
|
Jouberin (AHI1)
|
OT8K2YWY
|
AHI1_HUMAN
|
Increases Expression
|
[106] |
|
Leucine-rich repeat-containing protein 47 (LRRC47)
|
OTSE8NAO
|
LRC47_HUMAN
|
Decreases Expression
|
[13] |
|
RelA-associated inhibitor (PPP1R13L)
|
OTNCPLWE
|
IASPP_HUMAN
|
Increases Expression
|
[107] |
|
DNA repair and recombination protein RAD54-like (RAD54L)
|
OTEGMAKG
|
RAD54_HUMAN
|
Increases Expression
|
[14] |
|
Cytochrome c oxidase assembly factor 7 (COA7)
|
OTRQJYL6
|
COA7_HUMAN
|
Decreases Expression
|
[13] |
|
Mitochondrial potassium channel (CCDC51)
|
OTOJNHNA
|
MITOK_HUMAN
|
Decreases Expression
|
[13] |
|
Integrator complex subunit 15 (INTS15)
|
OT3O4INM
|
INT15_HUMAN
|
Decreases Expression
|
[13] |
|
Bcl-2-binding component 3, isoforms 3/4 (BBC3)
|
OTUAXDAY
|
BBC3B_HUMAN
|
Increases Expression
|
[53] |
|
Proheparin-binding EGF-like growth factor (HBEGF)
|
OTLU00JS
|
HBEGF_HUMAN
|
Increases Expression
|
[57] |
|
Krueppel-like factor 6 (KLF6)
|
OTQY9S7F
|
KLF6_HUMAN
|
Decreases Expression
|
[13] |
|
Mitogen-activated protein kinase kinase kinase 5 (MAP3K5)
|
OTQR6ENW
|
M3K5_HUMAN
|
Decreases Expression
|
[12] |
|
Cell division control protein 6 homolog (CDC6)
|
OTX93FE7
|
CDC6_HUMAN
|
Decreases Expression
|
[63] |
|
Growth/differentiation factor 15 (GDF15)
|
OTWQN50N
|
GDF15_HUMAN
|
Increases Expression
|
[63] |
|
SOSS complex subunit B1 (NABP2)
|
OTQU8081
|
SOSB1_HUMAN
|
Increases Acetylation
|
[108] |
|
Protein pelota homolog (PELO)
|
OTF3WLIM
|
PELO_HUMAN
|
Decreases Expression
|
[13] |
|
TIMELESS-interacting protein (TIPIN)
|
OT9PZHXV
|
TIPIN_HUMAN
|
Decreases Expression
|
[13] |
|
Cell cycle regulator of non-homologous end joining (CYREN)
|
OT5ZL00R
|
CYREN_HUMAN
|
Affects Localization
|
[109] |
|
Caspase recruitment domain-containing protein 10 (CARD10)
|
OT2RPM4I
|
CAR10_HUMAN
|
Increases Expression
|
[59] |
|
Angiotensin-converting enzyme 2 (ACE2)
|
OTTRZGU7
|
ACE2_HUMAN
|
Decreases Expression
|
[110] |
|
Sulfiredoxin-1 (SRXN1)
|
OTYDBO4L
|
SRXN1_HUMAN
|
Increases Expression
|
[111] |
|
tRNA pseudouridine(38/39) synthase (PUS3)
|
OT6WG6M2
|
PUS3_HUMAN
|
Decreases Expression
|
[13] |
|
Homeodomain-interacting protein kinase 2 (HIPK2)
|
OTDB7QC3
|
HIPK2_HUMAN
|
Increases Activity
|
[81] |
|
Serine/threonine-protein kinase PLK3 (PLK3)
|
OT19CT2Z
|
PLK3_HUMAN
|
Increases Expression
|
[12] |
|
Transmembrane protein 185B (TMEM185B)
|
OTGNYS0J
|
T185B_HUMAN
|
Decreases Expression
|
[13] |
|
Interferon-stimulated 20 kDa exonuclease-like 2 (ISG20L2)
|
OT3Q11WQ
|
I20L2_HUMAN
|
Decreases Expression
|
[13] |
|
GrpE protein homolog 1, mitochondrial (GRPEL1)
|
OT8O00YJ
|
GRPE1_HUMAN
|
Decreases Expression
|
[13] |
|
Tumor necrosis factor receptor superfamily member 12A (TNFRSF12A)
|
OT5V52LN
|
TNR12_HUMAN
|
Increases Expression
|
[59] |
|
Programmed cell death 1 ligand 1 (CD274)
|
OTJ0VFDL
|
PD1L1_HUMAN
|
Increases Expression
|
[112] |
|
Forkhead box protein J2 (FOXJ2)
|
OTPT639J
|
FOXJ2_HUMAN
|
Decreases Expression
|
[13] |
|
rRNA methyltransferase 2, mitochondrial (MRM2)
|
OTRYA1HU
|
MRM2_HUMAN
|
Decreases Expression
|
[13] |
|
DNA helicase MCM8 (MCM8)
|
OTC93H3S
|
MCM8_HUMAN
|
Decreases Expression
|
[63] |
|
Frizzled-1 (FZD1)
|
OTZATHVS
|
FZD1_HUMAN
|
Decreases Expression
|
[13] |
|
Calcium-binding protein 39 (CAB39)
|
OT2CL9ST
|
CAB39_HUMAN
|
Decreases Expression
|
[12] |
|
5'-AMP-activated protein kinase subunit beta-1 (PRKAB1)
|
OT1OG4QZ
|
AAKB1_HUMAN
|
Increases Expression
|
[13] |
|
Sestrin-1 (SESN1)
|
OTSFDZWL
|
SESN1_HUMAN
|
Increases Expression
|
[13] |
|
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
|
OT0HENJ5
|
NF2L2_HUMAN
|
Decreases Response To Substance
|
[113] |
|
Molybdenum cofactor sulfurase (MOCOS)
|
OT0TL3Q5
|
MOCOS_HUMAN
|
Affects Response To Substance
|
[18] |
|
Tripartite motif-containing protein 2 (TRIM2)
|
OT0V1YVC
|
TRIM2_HUMAN
|
Affects Response To Substance
|
[18] |
|
V-type proton ATPase 116 kDa subunit a 4 (ATP6V0A4)
|
OT149Z7Q
|
VPP4_HUMAN
|
Affects Response To Substance
|
[18] |
|
Insulin-like growth factor-binding protein 7 (IGFBP7)
|
OT1D416P
|
IBP7_HUMAN
|
Affects Response To Substance
|
[18] |
|
Acid ceramidase (ASAH1)
|
OT1DNGXL
|
ASAH1_HUMAN
|
Affects Response To Substance
|
[18] |
|
D-3-phosphoglycerate dehydrogenase (PHGDH)
|
OT1LMRTG
|
SERA_HUMAN
|
Affects Response To Substance
|
[18] |
|
Calcipressin-1 (RCAN1)
|
OT1MVXC7
|
RCAN1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Melanoma antigen recognized by T-cells 1 (MLANA)
|
OT1N2S2K
|
MAR1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Serine palmitoyltransferase small subunit A (SPTSSA)
|
OT2PNKOP
|
SPTSA_HUMAN
|
Affects Response To Substance
|
[18] |
|
Large neutral amino acids transporter small subunit 1 (SLC7A5)
|
OT2WPVXD
|
LAT1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Chitinase-3-like protein 1 (CHI3L1)
|
OT2Z7VJH
|
CH3L1_HUMAN
|
Decreases Response To Substance
|
[114] |
|
Syncytin-2 (ERVFRD-1)
|
OT3064D7
|
SYCY2_HUMAN
|
Affects Response To Substance
|
[18] |
|
DNA-directed RNA polymerase I subunit RPA12 (POLR1H)
|
OT30S3SB
|
RPA12_HUMAN
|
Decreases Response To Substance
|
[115] |
|
Protein FAM171A1 (FAM171A1)
|
OT33BHEP
|
F1711_HUMAN
|
Affects Response To Substance
|
[18] |
|
Fatty acid CoA ligase Acsl3 (ACSL3)
|
OT3MWER1
|
ACSL3_HUMAN
|
Affects Response To Substance
|
[18] |
|
Platelet-derived growth factor C (PDGFC)
|
OT3ZJ0XU
|
PDGFC_HUMAN
|
Affects Response To Substance
|
[18] |
|
Insulin receptor substrate 2 (IRS2)
|
OT46MJBE
|
IRS2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Nuclear protein 1 (NUPR1)
|
OT4FU8C0
|
NUPR1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Proteinase-activated receptor 1 (F2R)
|
OT4WVWBO
|
PAR1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Roundabout homolog 1 (ROBO1)
|
OT52UFVS
|
ROBO1_HUMAN
|
Affects Response To Substance
|
[18] |
|
DNA repair nuclease/redox regulator APEX1 (APEX1)
|
OT53OI14
|
APEX1_HUMAN
|
Decreases Response To Substance
|
[116] |
|
Lymphokine-activated killer T-cell-originated protein kinase (PBK)
|
OT5Z27TW
|
TOPK_HUMAN
|
Affects Response To Substance
|
[18] |
|
Protein E7 (E7)
|
OT6BIMFN
|
VE7_HPV16
|
Increases ADR
|
[52] |
|
G-protein-signaling modulator 2 (GPSM2)
|
OT6RPMRM
|
GPSM2_HUMAN
|
Affects Response To Substance
|
[18] |
|
CD40 ligand (CD40LG)
|
OT75Z6A6
|
CD40L_HUMAN
|
Decreases Response To Substance
|
[117] |
|
Monocarboxylate transporter 7 (SLC16A6)
|
OT7BIIZX
|
MOT7_HUMAN
|
Affects Response To Substance
|
[18] |
|
Homeobox protein engrailed-2 (EN2)
|
OT7EZCM2
|
HME2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Ras-related protein Rab-17 (RAB17)
|
OT7NBUGQ
|
RAB17_HUMAN
|
Affects Response To Substance
|
[18] |
|
Fibroblast growth factor 2 (FGF2)
|
OT7YUJ9F
|
FGF2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Myelin proteolipid protein (PLP1)
|
OT8CM9CX
|
MYPR_HUMAN
|
Affects Response To Substance
|
[18] |
|
RAC-alpha serine/threonine-protein kinase (AKT1)
|
OT8H2YY7
|
AKT1_HUMAN
|
Increases Response To Substance
|
[118] |
|
Phosphatidylinositol 4-phosphate 5-kinase type-1 beta (PIP5K1B)
|
OT8JQ1F7
|
PI51B_HUMAN
|
Affects Response To Substance
|
[18] |
|
Neuronal membrane glycoprotein M6-b (GPM6B)
|
OT8Q1582
|
GPM6B_HUMAN
|
Affects Response To Substance
|
[18] |
|
Melanoma-associated antigen 12 (MAGEA12)
|
OT8ULELL
|
MAGAC_HUMAN
|
Affects Response To Substance
|
[18] |
|
Solute carrier family 22 member 18 (SLC22A18)
|
OT9C3KR4
|
S22AI_HUMAN
|
Affects Response To Substance
|
[18] |
|
Thymosin beta-4 (TMSB4X)
|
OT9HPLP3
|
TYB4_HUMAN
|
Decreases Response To Substance
|
[119] |
|
Follicle-stimulating hormone receptor (FSHR)
|
OT9MVMLI
|
FSHR_HUMAN
|
Increases ADR
|
[120] |
|
Oxidoreductase HTATIP2 (HTATIP2)
|
OT9MZ4QO
|
HTAI2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Alpha-N-acetylgalactosaminide alpha-2,6-sialyltransferase 2 (ST6GALNAC2)
|
OT9PBQVT
|
SIA7B_HUMAN
|
Affects Response To Substance
|
[18] |
|
Splicing factor 45 (RBM17)
|
OT9ROJCL
|
SPF45_HUMAN
|
Decreases Response To Substance
|
[121] |
|
CD44 antigen (CD44)
|
OT9TTJ41
|
CD44_HUMAN
|
Affects Response To Substance
|
[122] |
|
Retinoblastoma tumor suppressor (RB1)
|
OT9VMY7B
|
Q5J3Q9_FUNHE
|
Increases ADR
|
[52] |
|
Tissue factor pathway inhibitor (TFPI)
|
OTA0FX16
|
TFPI1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Medium-chain specific acyl-CoA dehydrogenase, mitochondrial (ACADM)
|
OTA4P0FC
|
ACADM_HUMAN
|
Affects Response To Substance
|
[18] |
|
POM121 and ZP3 fusion protein (POMZP3)
|
OTAH2JOK
|
POZP3_HUMAN
|
Affects Response To Substance
|
[18] |
|
Transmembrane protein 47 (TMEM47)
|
OTAJ4CPX
|
TMM47_HUMAN
|
Affects Response To Substance
|
[18] |
|
Glutathione S-transferase theta-2 (GSTT2)
|
OTANW3TJ
|
GST2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Aldehyde dehydrogenase, dimeric NADP-preferring (ALDH3A1)
|
OTAYZZE6
|
AL3A1_HUMAN
|
Affects Response To Substance
|
[123] |
|
5-hydroxytryptamine receptor 4 (HTR4)
|
OTBIB44K
|
5HT4R_HUMAN
|
Affects Response To Substance
|
[18] |
|
High mobility group protein HMGI-C (HMGA2)
|
OTBMBHHL
|
HMGA2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Chondroitin sulfate N-acetylgalactosaminyltransferase 1 (CSGALNACT1)
|
OTBML9D9
|
CGAT1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Microtubule-associated tumor suppressor 1 (MTUS1)
|
OTBPALMU
|
MTUS1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Cystathionine gamma-lyase (CTH)
|
OTC90LA0
|
CGL_HUMAN
|
Affects Response To Substance
|
[18] |
|
Melanocyte protein PMEL (PMEL)
|
OTCDDHHM
|
PMEL_HUMAN
|
Affects Response To Substance
|
[18] |
|
BMP and activin membrane-bound inhibitor homolog (BAMBI)
|
OTCEJ8W5
|
BAMBI_HUMAN
|
Affects Response To Substance
|
[18] |
|
Alkaline phosphatase, germ cell type (ALPG)
|
OTCIM29R
|
PPBN_HUMAN
|
Affects Response To Substance
|
[18] |
|
Creatine kinase U-type, mitochondrial (CKMT1A)
|
OTCINHH5
|
KCRU_HUMAN
|
Affects Response To Substance
|
[18] |
|
Sodium/potassium-transporting ATPase subunit alpha-1 (ATP1A1)
|
OTCJ458Q
|
AT1A1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Ras-interacting protein 1 (RASIP1)
|
OTCRY2AN
|
RAIN_HUMAN
|
Affects Response To Substance
|
[18] |
|
Collagen alpha-3(IX) chain (COL9A3)
|
OTCUJOEK
|
CO9A3_HUMAN
|
Affects Response To Substance
|
[18] |
|
A-kinase anchor protein 12 (AKAP12)
|
OTCVRDDX
|
AKA12_HUMAN
|
Affects Response To Substance
|
[18] |
|
Interferon-stimulated gene 20 kDa protein (ISG20)
|
OTCWRJJW
|
ISG20_HUMAN
|
Affects Response To Substance
|
[18] |
|
Stathmin (STMN1)
|
OTDJ4RV0
|
STMN1_HUMAN
|
Affects Response To Substance
|
[124] |
|
Bcl-2 homologous antagonist/killer (BAK1)
|
OTDP6ILW
|
BAK_HUMAN
|
Increases Response To Substance
|
[125] |
|
Dickkopf-related protein 3 (DKK3)
|
OTDU94EY
|
DKK3_HUMAN
|
Affects Response To Substance
|
[18] |
|
P protein (OCA2)
|
OTDWIGBF
|
P_HUMAN
|
Affects Response To Substance
|
[18] |
|
Cystatin-SN (CST1)
|
OTE4I83Q
|
CYTN_HUMAN
|
Affects Response To Substance
|
[18] |
|
Alternative prion protein (PRNP)
|
OTE85L1Q
|
APRIO_HUMAN
|
Decreases Response To Substance
|
[126] |
|
Fructose-bisphosphate aldolase C (ALDOC)
|
OTEC13I5
|
ALDOC_HUMAN
|
Affects Response To Substance
|
[18] |
|
Interferon-induced transmembrane protein 1 (IFITM1)
|
OTECO1G8
|
IFM1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Epidermal growth factor-like protein 8 (EGFL8)
|
OTECTJ7K
|
EGFL8_HUMAN
|
Affects Response To Substance
|
[18] |
|
dTDP-D-glucose 4,6-dehydratase (TGDS)
|
OTEOFLOS
|
TGDS_HUMAN
|
Affects Response To Substance
|
[18] |
|
Mitogen-activated protein kinase 8 (MAPK8)
|
OTEREYS5
|
MK08_HUMAN
|
Increases ADR
|
[52] |
|
Ras association domain-containing protein 1 (RASSF1)
|
OTEZIPB7
|
RASF1_HUMAN
|
Increases Response To Substance
|
[127] |
|
Transcription factor SOX-10 (SOX10)
|
OTF25ULQ
|
SOX10_HUMAN
|
Affects Response To Substance
|
[18] |
|
Eukaryotic translation initiation factor 3 subunit A (EIF3A)
|
OTFABY9G
|
EIF3A_HUMAN
|
Increases Response To Substance
|
[128] |
|
Sortilin (SORT1)
|
OTFCQ4B1
|
SORT_HUMAN
|
Affects Response To Substance
|
[18] |
|
Kelch-like ECH-associated protein 1 (KEAP1)
|
OTFHOD0C
|
KEAP1_HUMAN
|
Increases Response To Substance
|
[113] |
|
Myelin basic protein (MBP)
|
OTFZCEDB
|
MBP_HUMAN
|
Affects Response To Substance
|
[18] |
|
Protein AF1q (MLLT11)
|
OTG5RVHC
|
AF1Q_HUMAN
|
Affects Response To Substance
|
[18] |
|
Tudor domain-containing protein 3 (TDRD3)
|
OTG83E2C
|
TDRD3_HUMAN
|
Affects Response To Substance
|
[18] |
|
Enhancer of filamentation 1 (NEDD9)
|
OTGCFN4M
|
CASL_HUMAN
|
Affects Response To Substance
|
[18] |
|
Squalene synthase (FDFT1)
|
OTGDISIT
|
FDFT_HUMAN
|
Affects Response To Substance
|
[18] |
|
Voltage-gated potassium channel subunit beta-2 (KCNAB2)
|
OTH115IE
|
KCAB2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Dual specificity protein phosphatase 2 (DUSP2)
|
OTH54FMR
|
DUS2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Sigma intracellular receptor 2 (TMEM97)
|
OTH76ZWK
|
SGMR2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Melanoma-associated antigen C1 (MAGEC1)
|
OTHMFHH8
|
MAGC1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Tribbles homolog 2 (TRIB2)
|
OTHSX3MX
|
TRIB2_HUMAN
|
Affects Response To Substance
|
[18] |
|
DNA damage-inducible transcript 4 protein (DDIT4)
|
OTHY8SY4
|
DDIT4_HUMAN
|
Affects Response To Substance
|
[18] |
|
BAG family molecular chaperone regulator 2 (BAG2)
|
OTI6LD27
|
BAG2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Nucleosome assembly protein 1-like 1 (NAP1L1)
|
OTI7WBZV
|
NP1L1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Melanoma-associated antigen 6 (MAGEA6)
|
OTI8S0O2
|
MAGA6_HUMAN
|
Affects Response To Substance
|
[18] |
|
Prosaposin receptor GPR37 (GPR37)
|
OTIMDDI3
|
GPR37_HUMAN
|
Affects Response To Substance
|
[18] |
|
Kynureninase (KYNU)
|
OTINL2RE
|
KYNU_HUMAN
|
Affects Response To Substance
|
[18] |
|
Lysosomal acid lipase/cholesteryl ester hydrolase (LIPA)
|
OTIOLNHE
|
LICH_HUMAN
|
Affects Response To Substance
|
[18] |
|
Baculoviral IAP repeat-containing protein 7 (BIRC7)
|
OTITCGHB
|
BIRC7_HUMAN
|
Decreases Response To Substance
|
[129] |
|
Runt-related transcription factor 3 (RUNX3)
|
OTITK1XD
|
RUNX3_HUMAN
|
Increases Response To Substance
|
[130] |
|
Protein S100-P (S100P)
|
OTJCXNJG
|
S100P_HUMAN
|
Increases Response To Substance
|
[131] |
|
Osteopontin (SPP1)
|
OTJGC23Y
|
OSTP_HUMAN
|
Affects Response To Substance
|
[18] |
|
cAMP-responsive element modulator (CREM)
|
OTJIJ5AL
|
CREM_HUMAN
|
Affects Response To Substance
|
[18] |
|
Alpha-(1,6)-fucosyltransferase (FUT8)
|
OTJJCVG1
|
FUT8_HUMAN
|
Affects Response To Substance
|
[18] |
|
Trefoil factor 3 (TFF3)
|
OTJJDRTU
|
TFF3_HUMAN
|
Affects Response To Substance
|
[18] |
|
Transcription factor-like 5 protein (TCFL5)
|
OTJL4348
|
TCFL5_HUMAN
|
Affects Response To Substance
|
[18] |
|
Uncharacterized protein C4orf19 (C4ORF19)
|
OTJMU7N6
|
CD019_HUMAN
|
Affects Response To Substance
|
[18] |
|
Sorbin and SH3 domain-containing protein 2 (SORBS2)
|
OTJSX44Y
|
SRBS2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Myc box-dependent-interacting protein 1 (BIN1)
|
OTK8O0X8
|
BIN1_HUMAN
|
Increases Response To Substance
|
[132] |
|
Transmembrane channel-like protein 6 (TMC6)
|
OTKH50J4
|
TMC6_HUMAN
|
Affects Response To Substance
|
[18] |
|
Cystine/glutamate transporter (SLC7A11)
|
OTKJ6PXW
|
XCT_HUMAN
|
Affects Response To Substance
|
[133] |
|
B-cell receptor-associated protein 31 (BCAP31)
|
OTKSACR4
|
BAP31_HUMAN
|
Increases ADR
|
[52] |
|
Myelin P2 protein (PMP2)
|
OTKYV2NE
|
MYP2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Cytochrome P450 26A1 (CYP26A1)
|
OTL1DFWV
|
CP26A_HUMAN
|
Decreases Response To Substance
|
[134] |
|
Histone-binding protein RBBP7 (RBBP7)
|
OTLB56HX
|
RBBP7_HUMAN
|
Increases ADR
|
[52] |
|
Glycylpeptide N-tetradecanoyltransferase 2 (NMT2)
|
OTLNA39Z
|
NMT2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Tyrosine-protein kinase Fyn (FYN)
|
OTLSLVZS
|
FYN_HUMAN
|
Affects Response To Substance
|
[18] |
|
Homeobox protein Hox-A5 (HOXA5)
|
OTLWGPQD
|
HXA5_HUMAN
|
Affects Response To Substance
|
[18] |
|
DNA cytosine-5)-methyltransferase 1 (DNMT1)
|
OTM2DGTK
|
DNMT1_HUMAN
|
Decreases Response To Substance
|
[135] |
|
Lymphocyte antigen 6E (LY6E)
|
OTMG16BZ
|
LY6E_HUMAN
|
Affects Response To Substance
|
[18] |
|
Bridging integrator 3 (BIN3)
|
OTMIWWDW
|
BIN3_HUMAN
|
Affects Response To Substance
|
[18] |
|
Bcl-2-like protein 2 (BCL2L2)
|
OTMKVO0J
|
B2CL2_HUMAN
|
Decreases Response To Substance
|
[136] |
|
Histone-lysine N-methyltransferase 2B (KMT2B)
|
OTMMAZQX
|
KMT2B_HUMAN
|
Increases ADR
|
[52] |
|
Copper-transporting ATPase 1 (ATP7A)
|
OTMR3GXP
|
ATP7A_HUMAN
|
Decreases Response To Substance
|
[137] |
|
Transcription factor AP-2-alpha (TFAP2A)
|
OTMYT3NK
|
AP2A_HUMAN
|
Increases Response To Substance
|
[138] |
|
Protein kinase C zeta type (PRKCZ)
|
OTN2FE42
|
KPCZ_HUMAN
|
Increases Response To Substance
|
[139] |
|
Four and a half LIM domains protein 1 (FHL1)
|
OTN535SU
|
FHL1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Epithelial splicing regulatory protein 1 (ESRP1)
|
OTNCS4SL
|
ESRP1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Beta-secretase 2 (BACE2)
|
OTO5YQVK
|
BACE2_HUMAN
|
Affects Response To Substance
|
[18] |
|
3',5'-cyclic-AMP phosphodiesterase 4B (PDE4B)
|
OTOA8WU2
|
PDE4B_HUMAN
|
Affects Response To Substance
|
[18] |
|
Hypoxanthine-guanine phosphoribosyltransferase (HPRT1)
|
OTOEEEXG
|
HPRT_HUMAN
|
Increases ADR
|
[52] |
|
Myeloperoxidase (MPO)
|
OTOOXLIN
|
PERM_HUMAN
|
Increases Metabolism
|
[140] |
|
Glutathione S-transferase Mu 4 (GSTM4)
|
OTOTF8DU
|
GSTM4_HUMAN
|
Affects Response To Substance
|
[18] |
|
Phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN (PTEN)
|
OTOWDUNT
|
PTEN_HUMAN
|
Increases Response To Substance
|
[62] |
|
Interleukin-4 (IL4)
|
OTOXBWAU
|
IL4_HUMAN
|
Decreases Response To Substance
|
[27] |
|
Ras-related protein Rab-20 (RAB20)
|
OTP9OOVS
|
RAB20_HUMAN
|
Affects Response To Substance
|
[18] |
|
DNA dC->dU-editing enzyme APOBEC-3C (APOBEC3C)
|
OTPL0AI1
|
ABC3C_HUMAN
|
Affects Response To Substance
|
[18] |
|
Epithelial membrane protein 2 (EMP2)
|
OTPS2H0L
|
EMP2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Ropporin-1A (ROPN1)
|
OTPXKZTX
|
ROP1A_HUMAN
|
Affects Response To Substance
|
[18] |
|
Complement component 1 Q subcomponent-binding protein, mitochondrial (C1QBP)
|
OTPYQX3K
|
C1QBP_HUMAN
|
Decreases Response To Substance
|
[141] |
|
Cyclic GMP-AMP phosphodiesterase SMPDL3A (SMPDL3A)
|
OTQDYH8E
|
ASM3A_HUMAN
|
Affects Response To Substance
|
[18] |
|
Histone deacetylase 4 (HDAC4)
|
OTQNGD32
|
HDAC4_HUMAN
|
Affects Response To Substance
|
[18] |
|
Serine/threonine-protein phosphatase 6 regulatory ankyrin repeat subunit A (ANKRD28)
|
OTRBREQ9
|
ANR28_HUMAN
|
Affects Response To Substance
|
[18] |
|
Bcl-2-like protein 1 (BCL2L1)
|
OTRC5K9O
|
B2CL1_HUMAN
|
Decreases Response To Substance
|
[142] |
|
Disabled homolog 2 (DAB2)
|
OTRMQTMZ
|
DAB2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Large ribosomal subunit protein eL6 (RPL6)
|
OTRU71O4
|
RL6_HUMAN
|
Decreases Response To Substance
|
[143] |
|
Band 4.1-like protein 3 (EPB41L3)
|
OTS6CHG2
|
E41L3_HUMAN
|
Affects Response To Substance
|
[18] |
|
Glutathione S-transferase Mu 1 (GSTM1)
|
OTSBF2MO
|
GSTM1_HUMAN
|
Affects Response To Substance
|
[28] |
|
Heat shock-related 70 kDa protein 2 (HSPA2)
|
OTSDET7B
|
HSP72_HUMAN
|
Affects Response To Substance
|
[18] |
|
MyoD family inhibitor domain-containing protein (MDFIC)
|
OTSRYBWZ
|
MDFIC_HUMAN
|
Affects Response To Substance
|
[18] |
|
Terminal nucleotidyltransferase 5A (TENT5A)
|
OTSYF511
|
TET5A_HUMAN
|
Affects Response To Substance
|
[18] |
|
Collagen alpha-1(XV) chain (COL15A1)
|
OTTFKK18
|
COFA1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Bcl-2-interacting killer (BIK)
|
OTTH1T3D
|
BIK_HUMAN
|
Increases Response To Substance
|
[144] |
|
Serine/threonine-protein kinase Chk1 (CHEK1)
|
OTTTI622
|
CHK1_HUMAN
|
Affects Response To Substance
|
[145] |
|
Max-interacting protein 1 (MXI1)
|
OTUQ9E0D
|
MXI1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Calcium-dependent secretion activator 2 (CADPS2)
|
OTV1FW0M
|
CAPS2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Forkhead box protein F2 (FOXF2)
|
OTV20NGX
|
FOXF2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Metastasis-associated in colon cancer protein 1 (MACC1)
|
OTV3DLX0
|
MACC1_HUMAN
|
Increases ADR
|
[120] |
|
Glutathione S-transferase LANCL1 (LANCL1)
|
OTVCMZXB
|
LANC1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Matrilin-2 (MATN2)
|
OTVDR68G
|
MATN2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Microtubule-actin cross-linking factor 1, isoforms 1/2/3/4/5 (MACF1)
|
OTVIHD77
|
MACF1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Poly polymerase tankyrase-1 (TNKS)
|
OTVOUTPX
|
TNKS1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Ensconsin (MAP7)
|
OTVSSJ33
|
MAP7_HUMAN
|
Affects Response To Substance
|
[18] |
|
Integrin beta-3 (ITGB3)
|
OTWCK1K6
|
ITB3_HUMAN
|
Increases Response To Substance
|
[146] |
|
N-myc proto-oncogene protein (MYCN)
|
OTWD33K1
|
MYCN_HUMAN
|
Decreases Response To Substance
|
[147] |
|
Sorbin and SH3 domain-containing protein 1 (SORBS1)
|
OTWH8762
|
SRBS1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Anamorsin (CIAPIN1)
|
OTWS90F9
|
CPIN1_HUMAN
|
Decreases Response To Substance
|
[148] |
|
Inositol 1,4,5-trisphosphate receptor type 1 (ITPR1)
|
OTX7MWW1
|
ITPR1_HUMAN
|
Affects Response To Substance
|
[18] |
|
S-adenosylhomocysteine hydrolase-like protein 1 (AHCYL1)
|
OTX8L3M5
|
SAHH2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Long-chain fatty acid transport protein 3 (SLC27A3)
|
OTXB60L7
|
S27A3_HUMAN
|
Affects Response To Substance
|
[18] |
|
Insulin-like growth factor 1 receptor (IGF1R)
|
OTXJIF13
|
IGF1R_HUMAN
|
Affects Response To Substance
|
[149] |
|
Estrogen receptor beta (ESR2)
|
OTXNR2WQ
|
ESR2_HUMAN
|
Increases Response To Substance
|
[150] |
|
Coiled-coil domain-containing protein 6 (CCDC6)
|
OTXRQDYG
|
CCDC6_HUMAN
|
Decreases Response To Substance
|
[151] |
|
Arginine vasopressin-induced protein 1 (AVPI1)
|
OTXSBR60
|
AVPI1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Carbamoyl-phosphate synthase , mitochondrial (CPS1)
|
OTXV8NSR
|
CPSM_HUMAN
|
Affects Response To Substance
|
[18] |
|
Peripheral myelin protein 22 (PMP22)
|
OTXWYWCZ
|
PMP22_HUMAN
|
Affects Response To Substance
|
[18] |
|
SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily E member 1-related (HMG20B)
|
OTY94WRA
|
HM20B_HUMAN
|
Affects Response To Substance
|
[18] |
|
Protein Niban 1 (NIBAN1)
|
OTYOLI12
|
NIBA1_HUMAN
|
Affects Response To Substance
|
[18] |
|
LIM and calponin homology domains-containing protein 1 (LIMCH1)
|
OTYPC4XA
|
LIMC1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Galectin-3 (LGALS3)
|
OTYQT0ZI
|
LEG3_HUMAN
|
Affects Response To Substance
|
[18] |
|
L-dopachrome tautomerase (DCT)
|
OTYVNTBG
|
TYRP2_HUMAN
|
Affects Response To Substance
|
[18] |
|
PRA1 family protein 3 (ARL6IP5)
|
OTYZ6BEQ
|
PRAF3_HUMAN
|
Increases Response To Substance
|
[152] |
|
DNA (DNMT3B)
|
OTZ0JCNP
|
DNM3B_HUMAN
|
Decreases Response To Substance
|
[135] |
|
Calcitonin (CALCA)
|
OTZ11LHB
|
CALC_HUMAN
|
Decreases Response To Substance
|
[153] |
|
Hephaestin (HEPH)
|
OTZ2F15Z
|
HEPH_HUMAN
|
Affects Response To Substance
|
[18] |
|
Complement decay-accelerating factor (CD55)
|
OTZ3HWTO
|
DAF_HUMAN
|
Affects Response To Substance
|
[18] |
|
Tissue factor pathway inhibitor 2 (TFPI2)
|
OTZCRWOR
|
TFPI2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Insulin-induced gene 1 protein (INSIG1)
|
OTZF5X1D
|
INSI1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Suppressor of tumorigenicity 7 protein (ST7)
|
OTZG8RC6
|
ST7_HUMAN
|
Affects Response To Substance
|
[18] |
|
UPF0489 protein C5orf22 (C5ORF22)
|
OTZN0DCX
|
CE022_HUMAN
|
Affects Response To Substance
|
[18] |
|
Alkaline phosphatase, placental type (ALPP)
|
OTZU4G9W
|
PPB1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Drebrin (DBN1)
|
OTZVKG8A
|
DREB_HUMAN
|
Affects Response To Substance
|
[18] |
| ------------------------------------------------------------------------------------ |
|
|
|
|